Advertisements
Advertisements
Question
Define carbolic acid.
Solution
Phenols are organic aromatic, hydroxyl compounds, in which one or more hydroxyl (-OH)groups are directly attached to the aromatic nucleus (i.e., benzene like ring).
APPEARS IN
RELATED QUESTIONS
Write the molecular and structural formula of BHA and BHT.
How carbolic acid is prepared from benzene sulphonic acid ?
Give one chemical test to distinguish between the following pair of compounds: Acetone and phenol.
How will you obtain the following? (Give balanced equation.)
Picric acid from phenol.
How will you obtain the following? (Give balanced equation.)
(i) Anisole from phenol
(ii) Ethyl acetate from ethanol.
What is the product A obtained in the following reaction?
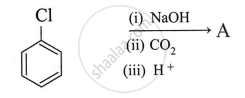
Identify product in:
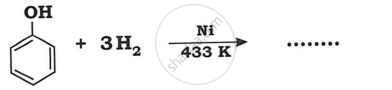
Identify product in:
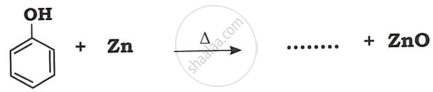
Match the following:
| (i) | Phenol | (a) | Hexane + heptane |
| (ii) | EDTA | (b) | Globular protein |
| (iii) | Ideal solution | (c) | Azo dye |
| (iv) | Insulin | (d) | Hexadentate ligand |
Account for the following:
Phenol is a stronger acid than aliphatic alcohol.
Write a chemical test to distinguish between ethanol and phenol.
