Advertisements
Advertisements
Questions
Derive an expression for maximum work in isothermal reversible expansion of two moles of an ideal gas.
Derive an expression for maximum work obtainable during isothermal reversible expansion of an ideal gas from initial volume (V1) to final volume (V2).
Solution
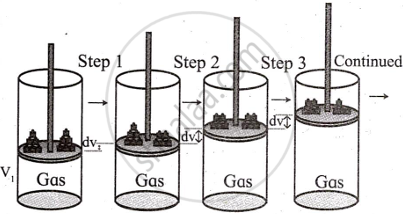
- Consider the expansion of ‘n’ moles of an ideal gas enclosed in a cylinder fitted with a weightless and
frictionless piston at a constant temperature T. - Let the pressure of the gas be P, which is equal to the external pressure.
- Let the external pressure be reduced by an infinitesimally small amount dp so that the new external pressure becomes (P − dp).
- Let the corresponding small increase in volume by dv.
- Therefore, the amount of work done in the expansion of the gas, dw = −[(p dp)dv] = −[pdv − dp.dv] ..(i)
- Since dp and dv are very small, their product (dp.dv) will be much smaller and can be neglected ∴ dw = −pdv ...(ii)
-
During expansion of the gas, if the initial volume V1 changes to volume V2 then total amount of work alone (Wmax) can be obtained by integrating the work, dw between the two limits, V1 and V2.
`therefore "W"_"max" = int_("v"_2)^("v"_1) - dw`
= `int_("v"_1)^("v"_2) - pdv` ...(iii)
But pv = nRT (Ideal gas Equation for n moles of a gas)
`therefore "p" = "nRT"/"v"`
Substitute pressure value in equation (iii)
`therefore "W" _"max" = int_("v"_1)^("v"_2) - ("nRT")/"v" "dv"`
= `"nRT" int_("v"_1)^("v"_2) "dv"/"v"` (n,R,T are constant)
= `-"nRT" ["log v"]_("v"_1)^("v"_2)`
= `-"nRT" "ln" "v"_2/"v"_1`
`therefore "W"_"max" = -2.303 "nRT" "log" "v"_2/"v"_1`
(In = 2.303 log10) ...(iv)
RELATED QUESTIONS
Write mathematical equation of first law of thermodynamics for the following processes :
Adiabatic process.
Write mathematical equation of first law of thermodynamics for Isochoric process.
Write one statement of first law of thermodyamics and its mathematical expression.
Prove that ΔH=ΔU+ΔnRT. what is the condition under which ΔU=ΔH?
Calculate the internal energy at 298K for the formation of one mole of ammonia, if the enthalpy change at constant pressure is – 42.0 kJ mol-1.
(Given: R = 8.314 J K-1 mol-1)
An ideal monoatomic gas is adiabatically compressed so that its final temperature is twice its initial temperature. What is the ratio of the final pressure to its initial pressure?
