HSC Science (General)
HSC Science (Electronics)
HSC Science (Computer Science)
Academic Year: 2023-2024
Date & Time: 29th February 2024, 11:00 am
Duration: 3h
Advertisements
General Instructions:
The question paper is divided into four Sections.
- Section A:
Q. No. 1 contains Ten multiple choice type of questions carrying One mark each. Only the first attempt will be considered for evaluation.
Q. No. 2 contains Eight very short answer type questions carrying One mark each. - Section B: Q. No. 3 to Q. No. 14 are Twelve short answer type questions carrying Two marks each. (Attempt any Eight)
- Section C: Q. No. 15 to Q. No. 26 are Twelve short answer type questions carrying Three marks each. (Attempt any Eight)
- Section D: Q. No. 27 to Q. No. 31 are Five long answer type questions carrying Four marks each. (Attempt any Three)
- Use of log table is allowed. Use of calculator is not allowed.
- Figures to the right indicate full marks.
- Given: R = 8.314 J.K-1.mol-1
NA= 6.022 x 1023
F = 96500C
. ade
The spin only magnetic moment of Cr3+ cation is ______.
3.742 BM
3.755 BM
3.873 BM
3.633 BM
Chapter: [0.08] Transition and Inner Transition Elements
The linkage present in Lactose is ______.
α, β - 1, 2 - glycosidic linkage
α - 1, 4 - glycosidic linkage
β - 1, 4 - glycosidic linkage
α - 1, 4 - glycosidic linkage
Chapter: [0.14] Biomolecules
The product of the following reaction is
\[\begin{array}{cc}
\ce{O}\phantom{.........}\\
||\phantom{.........}\\
\ce{C2H5 - C - CH3 ->[H2/Ni][\Delta] \phantom{..}?}\end{array}\]
\[\ce{CH3 - CH2 - CH2 - CH2 - OH}\]
\[\begin{array}{cc}
\ce{CH3 - CH - CH2 - CH3}\\
|\phantom{.........}\\
\ce{OH}\phantom{.......}
\end{array}\]
\[\ce{CH3 - CH2 - CH2 - CH3}\]
\[\ce{CH3 - CH2 - CH2 - COOH}\]
Chapter: [12.01] Aldehydes and Ketones
The pH of 0.001 M HCl solution is ______.
1.0
2.0
3.0
7.0
10
11
Chapter: [0.03] Ionic Equilibria
The correct structure of complex having IUPAC name sodium hexanitrocobaltate (III) is ______.
\[\ce{Na3[Co(NO2)5]}\]
\[\ce{Na4[Co(NO2)6]}\]
\[\ce{Na3[Co(NO2)6]}\]
\[\ce{Na4[Co(NO2)5]}\]
Chapter: [0.09] Coordination Compounds
The number of particles present in Face Centred Cubic Unit cell is/are ______.
1
2
3
4
Chapter: [0.01] Solid State
The monomer used in preparation of teflon is ______.
E caprolactum
vinyl chloride
styrene
tetrafluoroethylene/tetrafluoroethene
Chapter: [0.15] Introduction to Polymer Chemistry [0.15] Polymers
Among the following vinylic halide is ______.
\[\begin{array}{cc}\ce{R - CH - R}\\|\phantom{..}\\\ce{X}\phantom{..}\end{array}\]
\[\ce{CH2 = CH - X}\]
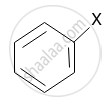
\[\ce{CH2 = CH - CH2 - X}\]
Chapter: [0.1] Halogen Derivatives
The product of hydrolysis of propyne in the presence of 1% \[\ce{HgSO4}\] and 40% \[\ce{H2SO4}\] is ______.
methanal
ethanal
Propanal
propanone
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids
If unit of rate constant is mol dm−3s−1, the order of reaction would be ______.
zero
1
2
3
Chapter: [0.06] Chemical Kinetics
Write the name of metal nanoparticle used to remove E.coli bacteria from water.
Chapter: [0.16] Green Chemistry and Nanochemistry
Write the name of reduction product formed when ethyl cyanide is treated with sodium and alcohol.
Chapter: [0.13] Amines [13.01] Amines
Complete the reaction:
\[\ce{CH3CH2Cl ->[AgCN][alc.\Delta]}\] ?
Chapter: [0.1] Halogen Derivatives
Calculate effective atomic number of \[\ce{[Co(NH3)6]^3+}\] ion.
Chapter: [0.09] Coordination Compounds
The compounds of \[\ce{Ti^4+}\] ions are colourless due to ______.
Chapter: [8.01] D-block Elements
What is the SI unit of molar conductivity?
Chapter: [0.05] Electrochemistry
Write the sign convention of work done during expansion of gas.
Chapter: [0.04] Chemical Thermodynamics
Write the condition of reverse osmosis.
Chapter: [0.02] Solutions [0.02] Solutions and Colligative Properties
Derive an expression for maximum work in isothermal reversible expansion of two moles of an ideal gas.
Chapter: [0.03] Chemical Thermodynamics and Energetic
What are interhalogen compounds?
Chapter: [0.07] Elements of Groups 16, 17 and 18
Write the chemical reaction when chlorine reacts with dry slaked lime.
Chapter: [0.07] Elements of Groups 16, 17 and 18 [7.03] Group 17 Elements
What is nano material?
Chapter: [0.16] Green Chemistry and Nanochemistry
Write the reaction involved in sol-gel process during hydrolysis.
Chapter: [0.16] Green Chemistry and Nanochemistry
Write a classification of proteins with an example.
Chapter: [14.02] Proteins
Calculate the time required to deposit 2.4 g of \[\ce{Cu}\], when 2.03 A of current passed through \[\ce{CuSO4}\], solution. (At. mass of \[\ce{Cu}\] = 63.5 g mol−1)
Chapter: [0.04] Chemical Thermodynamics
Among dimethylamine (pKb = 3.27) and diethylamine (pKb = 3.0), which one is more basic?
Chapter: [13.01] Amines
Explain buffer action of sodium acetate-acetic acid buffer.
Chapter: [0.03] Ionic Equilibria
Advertisements
Write preparation of diethyl ether.
Chapter: [0.11] Alcohols, Phenols and Ethers
Write preparation of ethyl cyanide from ethyl bromide.
Chapter: [13.02] Cyanides and Isocyanides
Henry's constant for \[\ce{CH3Br_{(g)}}\] is 0.159 mol dm−3 bar−1 25°C. Calculate its solubility in water at 25°C, if its partial pressure is 0.164 bar.
Chapter: [0.02] Solutions
Write the structure and name of monomer of Nylon-6.
Chapter: [0.15] Introduction to Polymer Chemistry [0.15] Polymers
Write the structure and name of monomer of Natural rubber.
Chapter: [0.15] Introduction to Polymer Chemistry [0.15] Polymers
Define lanthanoid contraction.
Chapter: [8.02] F-block Elements
Write the balanced chemical equations when acidified \[\ce{K2Cr2O7}\] reacts with \[\ce{H2S}\].
Chapter: [0.08] Transition and Inner Transition Elements
Obtain the relationship between the density of a substance and the edge length of the unit cell.
Chapter: [0.01] Solid State
Answer the following in one or two sentences.
What is osmotic pressure?
Chapter: [0.02] Solutions [0.02] Solutions and Colligative Properties
How will you determine molar mass of solute from osmotic pressure?
Chapter: [0.02] Solutions [0.02] Solutions and Colligative Properties
Write chemical equation for the following :
Rosenmund reduction
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids [12.01] Aldehydes and Ketones
Write chemical equation for the following:
Gatterman - Koch formylation
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids [12.01] Aldehydes and Ketones
Write the chemical reaction involved in Cannizzaro reaction of methanal.
Chapter: [12.01] Aldehydes and Ketones
Calculate the standard enthalpy of combustion of methane if the standard enthalpy of formation of methane, carbon dioxide and water are −74.8, −393.5 and −285.8 kJmol−1 respectively.
Chapter: [0.04] Chemical Thermodynamics
What is the action of following on ethyl bromide?
silver nitrite
Chapter: [0.1] Halogen Derivatives
What is the action of following on ethyl bromide?
\[\ce{Mg}\] in dry ether
Chapter: [0.1] Halogen Derivatives
What is the action of following on ethyl bromide?
alcoholic sodium hydroxide
Chapter: [0.1] Halogen Derivatives
For the reaction A + B → P.
If [B] is doubled at constant [A], the rate of reaction doubled. If [A] is triple and [B] is doubled, the rate of reaction increases by a factor of 6. Calculate the rate law equation.
Chapter: [0.06] Chemical Kinetics
Arrange the following in the increasing order of the property mentioned:
\[\ce{HOCl}\], \[\ce{HClO2}\], \[\ce{HClO3}\], \[\ce{HClO4}\] (acidic strength)
Chapter: [0.07] Elements of Groups 16, 17 and 18
Arrange the following in the increasing order of the property mentioned:
\[\ce{MF}\], \[\ce{MCl}\], \[\ce{MBr}\], \[\ce{MI}\] (ionic character)
Chapter: [7.03] Group 17 Elements
Arrange the following in the increasing order of the property mentioned:
\[\ce{HF}\], \[\ce{HCl}\], \[\ce{HBr}\], \[\ce{HI}\] (thermal stability)
Chapter: [0.07] Elements of Groups 16, 17 and 18
Explain Wolf-Kishner reduction reaction.
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids
Write preparation of propanone by using ethanoyl chloride and dimethyl cadmium.
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids [12.01] Aldehydes and Ketones
Write postulates of Werner theory of co-ordination complexes.
Chapter: [0.09] Coordination Compounds
Write the name of a hexadentate ligand.
Chapter: [0.09] Coordination Compounds
Advertisements
Define electrochemical series.
Chapter: [0.05] Electrochemistry
Write applications of electrochemical series.
Chapter: [0.05] Electrochemistry
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids
Define acids and bases according to Bronsted-Lowry theory.
Chapter: [0.03] Ionic Equilibria
Derive the relationship between pH and pOH.
Chapter: [0.03] Ionic Equilibria
Write reactions involved in preparation of potassium dichromate from chrome iron ore
Chapter: [0.08] Transition and Inner Transition Elements
Convert the following:
acetaldehyde to isopropyl alcohol.
Chapter: [11.01] Alcohols
Convert the following :
cumene to phenol.
Chapter: [0.11] Alcohols, Phenols and Ethers
Convert the following :
anisole to phenol.
Chapter: [11.03] Ethers
Write two uses of neon.
Chapter: [0.07] Elements of Groups 16, 17 and 18 [7.04] Group 18 Elements
Define the Extensive properties.
Chapter: [0.04] Chemical Thermodynamics
Define Intensive property.
Chapter: [0.04] Chemical Thermodynamics
Define Adiabatic process.
Chapter: [0.04] Chemical Thermodynamics
Write the atomic numbers of transuranium elements.
Chapter: [0.08] Transition and Inner Transition Elements
Predict the type of cubic lattice of a solid element having edge length of 400 pm and density is 6.25 g/ml.
(Atomic mass of element = 60)
Chapter: [0.01] Solid State
Define the following term:
Nanoscience
Chapter: [0.16] Green Chemistry and Nanochemistry
Write a chemical reaction for the preparation of the following polymer.
polyacrylonitrile
Chapter: [0.15] Introduction to Polymer Chemistry [0.15] Polymers
Derive an expression for the relation between half-life and rate constant for first-order reaction.
Chapter: [0.06] Chemical Kinetics
Write the net cell reaction during discharging of lead accumulator.
Chapter: [0.05] Electrochemistry
Draw the structure of peroxymonosulphuric acid.
Chapter: [0.07] Elements of Groups 16, 17 and 18
Mention the number of unpaired electrons and geometry of the following complex:
\[\ce{[Ni(Cl)4]^{2-}}\]
Chapter: [0.09] Coordination Compounds
Mention the number of unpaired electrons and geometry of the following complex:
\[\ce{[Ni(CN)4]^2-}\]
Chapter: [0.09] Coordination Compounds
Convert the following:
Ethanenitrile into ethanal.
Chapter:
Convert the following:
Cyclohexene into adipic acid.
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
Maharashtra State Board previous year question papers 12th Standard Board Exam Chemistry with solutions 2023 - 2024
Previous year Question paper for Maharashtra State Board 12th Standard Board Exam Chemistry-2024 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of Maharashtra State Board 12th Standard Board Exam.
How Maharashtra State Board 12th Standard Board Exam Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
