Advertisements
Advertisements
Question
Describe simple experiments to show the presence of -
oxygen and nitrogen component in air using a bell jar
Solution
Oxygen and nitrogen in air:
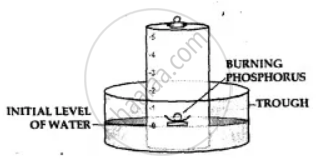
Apparatus— A through, a bell jar, a cork and a crucible and a piece of white phosphorus.
producer-
- The trough is filled with water and a bell jar divided into 5 equal parts, ‘1 to 5’ is placed over it.
- A crucible containing white phosphorus is placed on a cork which is made to float on water.
- The level of the water inside and outside are adjusted to one level.
- The phosphorus is then ignited by means of a heated wire.
Observation-
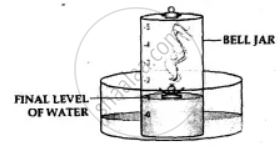
- The phosphorus burns in the active component of air [oxygen] forming – dense white fumes of phosphorus pentoxide [P2O5]. Phosphorus + Oxygen →Phosphorus pentoxide
- The level of the water in the bell jar rises by l/5th.
Conclusion-
- Oxygen— The active component of air, i.e. 1/5th of air, is used up in burning.
- Nitrogen— The inactive component of air, i.e. 4/ 5 of air, is not used up in burning. Hence air contains oxygen which supports combustion and nitrogen which does not.
APPEARS IN
RELATED QUESTIONS
What is rust ?
The process by which oxidation of food in our body takes place is
Write ‘True’ or ‘False’ in front of following statement.
Nitrogen is a gaseous non-metal essential for respiration.
What happens when Iron burns in oxygen ? Also give the balanced chemical equation for the reaction.
What is rusting?
Identify the acidic oxides responsible for acid rain. State how their presence results in formation of acid rain. Give a reason why acid rain damages heritage buildings.
In the laboratory preparation of oxygen from hydrogen peroxide answer the following:
State the method of collection of the oxygen gas giving reasons.
Give reason for the following:
Combustion and respiration show similarity.
In Rockets, along with fuel, oxygen is also carried for combustion – why?
A process without emitting flame is called as ______.
