Advertisements
Advertisements
Question
Draw an electron dot diagram to show the formation of each of the following compounds:
Magnesium Chloride
[H = 1, C = 6, Mg = 12, Cl = 17]
Solution
Magnesium atom loses 2 electrons to attain a stable electronic configuration and becomes a cation.
Example:
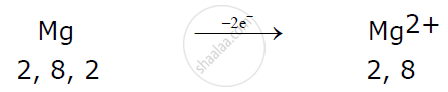
A non-metallic atom like chlorine gains 1 electron to attain a stable electronic configuration and becomes an anion.
Example:
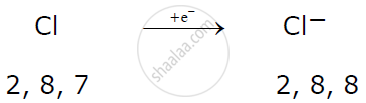
Cations and anions are oppositely charged particles which attract one another to form an electrovalent bond leading to the formation of an electrovalent compound
Here magnesium donates one electron each with two chlorine atoms resulting in the formation of magnesium chloride.

APPEARS IN
RELATED QUESTIONS
Define the term ‘electron affinity’.
Arrange the elements of second period in increasing order of their electron affinity. Name the elements which do not follow the trend in this period.
A, B, C are three elements in which B is an inert gas other than helium.With this information complete the following table.
| Element | Atomic number | No. of electrons in the valence shell | Group to which the element belongs |
| A | Z - 1 | ||
| B | Z | ||
| C | Z + 1 |
Also, explain the following : Electron affinity of B is Zero.
What is electron affinity?
With reference to the variation of properties in the Periodic Table, which of the following is generally true?
Electron affinity increases going down a group.
Down the group, electron affinity ______.
The electron affinity of an element X is greater than that of element Y.
How is the electronegativity of X likely to compare with that of Y?
Arrange the following as per instruction given in the bracket.
Cl, F, Br, I (increasing electron affinity)
Among period 2 elements A, B, C and D, the one which has highest electron affinity is ______.
