Advertisements
Advertisements
Question
Draw neat labelled diagrams for two different experiments to prove that – hydrogen is lighter than air.
Solution
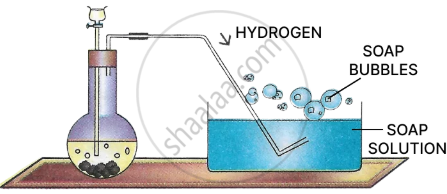
Experiment 1
Procedure: Bubble hydrogen gas by means of a small pipe into a dish containing soap solution.
Observation: Soap bubbles filled with H2 rise upwards.
Conclusion: Hydrogen is lighter than air hence soap bubbles filled with hydrogen rise up.
Experiment 2
Procedure: Take two dry test tubes.
Test tube 'A' - Filled with hydrogen.
Test tube 'B' - Filled with air.
Place test tube 'B' over test tube 'A' and test the gas in the upper test tube 'B' with a glowing splinter.
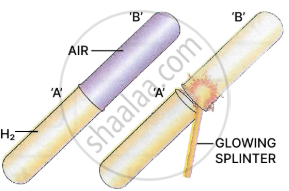
Observation: A 'pop' sound is heard in test tube 'B'. [hydrogen burns with a 'pop' sound].
Conclusion: Hydrogen is lighter than air and has moved upwards displacing the air.
APPEARS IN
RELATED QUESTIONS
Name the following:
A metal which liberates hydrogen only when steam is passed over red hot metal.
Name the following:
A metallic oxide which can be reduced into metal by hydrogen.
Name the chemicals required to prepare hydrogen gas in the laboratory.
Name the impurities present in hydrogen prepared in the laboratory.
Starting from zinc how would you obtain hydrogen using Steam.
[Give balanced equation & name the product formed in the case other than hydrogen].
Name a metal which will not react with the reactants above to give hydrogen.
In the laboratory preparation of hydrogen from zinc & dilute hydrocholoric acid – state a reason for the collecting the hydrogen by downward displacement of water and not air & collecting it after all the air in the apparatus is allowed to escape.
Give reason for the following:
In the laboratory preparation of hydrogen from zinc and dilute hydrochloric acid – the zinc used granulated zinc.
In the laboratory preparation of hydrogen from zinc and dil. acid. Give a reason for the following:
The complete apparatus is air-tight.
In the laboratory preparation of hydrogen from zinc and dil. acid. Give a reason for the following:
Dilute nitric acid is not preferred as the reactant acid.
Give a reason for the following.
Nitric acid in the dilute form is not used in the laboratory preparation of hydrogen from metals.
