Advertisements
Advertisements
Question
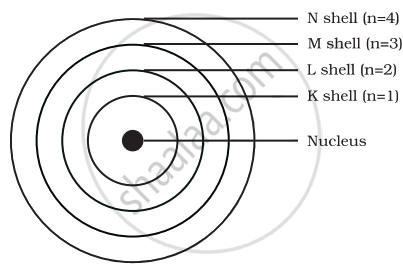
Draw a sketch of Bohr’s model of an atom with three shells.
Solution
According to Bohr's atomic model with three orbitals, the order of the three orbitals will be K, L and M
The number of electrons in each orbit = 2n2
Number of electrons in K orbit = 2 × 12 = 2
Number of electrons in L orbit = 2 × 22 = 8
Number of electrons in M orbit = 2 × 32 = 18
But the maximum number of electrons in the outer orbit can be 8.
APPEARS IN
RELATED QUESTIONS
Describe Bohr’s model of the atom.
Compare all the proposed models of an atom given in this chapter.
Name the scientists who described the arrangement of electrons in an atom.
What are the various letters used by Bohr to represent electron shells in an atom?
State true or false. If false, correct the statement.
Smaller the size of the orbit, lower is the energy of the orbit.
Explain the postulates of Bohr’s atomic model.
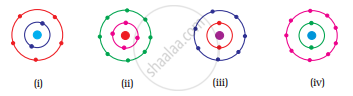
Which of the following in Fig. 4.2 do not represent Bohr’s model of an atom correctly?
Niels Bohr was Born on ______.
The atomic model based on quantum theory was first proposed by ______.
The scientist who proposed the atomic model based on the quantisation of energy for the first time is ______.
