Advertisements
Advertisements
Question
Draw the structure of BrF5
Solution
BrF5
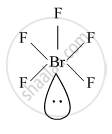
shaalaa.com
Is there an error in this question or solution?
2015-2016 (March) Delhi Set 1
APPEARS IN
RELATED QUESTIONS
Explain the structure of ClF3
Draw the structures of the following: BrF5
Find the INCORRECT match.
What is the oxidation state of bromine in the product?
\[\ce{Br2 + \underset{(excess)}{3F2} ->?}\]
The CORRECT statement about bromine trifluoride is:
The bent T-shaped interhalogen is ____________.
BrF undergoes disproportionation to form Br2 and another interhalogen compound. What is the oxidation state of Br in this interhalogen compound?
Which among the following halogen does not form polyhalide ion?
What type of hybridization is observed in interhalogen compounds of the type \[\ce{XX^'_3}\]?
In the interhalogen compound AB3, the state of hydridisation of A is ______.
