Advertisements
Advertisements
Question
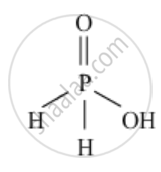
Draw the structures of `H_3PO_2`
Solution
`H_3PO_2`
APPEARS IN
RELATED QUESTIONS
Give reasons for the following : Oxygen has less electron gain enthalpy with negative sign than sulphur.
Which of the following does not react with oxygen directly?
Zn, Ti, Pt, Fe
Arrange the following in the order of property indicated for each set:
F2, Cl2, Br2, I2 - increasing bond dissociation enthalpy.
Arrange the following in the order of the property indicated against set :
H2O, H2S, H2Se, H2Te − increasing acidic character.
Give reactions for the following:
O – O single bond is weaker than S – S single bond.
Match the items of Columns I and II and mark the correct option.
| Column I | Column II |
| (A) \[\ce{H2SO4}\] | (1) Highest electron gain enthalpy |
| (B) \[\ce{CCl3NO2}\] | (2) Chalcogen |
| (C) \[\ce{Cl2}\] | (3) Tear gas |
| (D) Sulphur | (4) Storage batteries |
Given below are two statements labelled as Assertion (A) and Reason (R).
Assertion (A): Electron gain enthalpy of oxygen is less than that of Flourine but greater than Nitrogen.
Reason (R): Ionisation enthalpies of the elements follow the order Nitrogen > Oxygen > Fluorine.
Select the most appropriate answer from the options given below:
Out of \[\ce{H2O}\] and \[\ce{H2S}\], which one has higher bond angle and why?
The correct order of ΔiHs among the following elements is
These are physical properties of an elements.
- Sublimation enthalpy
- Ionisation enthalpy
- Hydration enthalpy
- Electron gain enthalpy
The total number of above properties that affect the reduction potential is ______. (Integer answer)
