Advertisements
Advertisements
Question
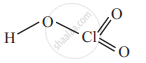
Draw the structures of the HClO3.
Solution
Chloric acid, HClO3
APPEARS IN
RELATED QUESTIONS
Write chemical formula of the following oxoacids of chlorine:
a. Hypochlorous acid
b. Chlorous acid
c. Chloric acid
d. Perchloric acid
Account for the following :
Fluorine forms only one oxoacid HOF
Draw the structures of the following:
(1) HClO4
(2) H3PO3
Account for the following :
HClO4 is a stronger acid than HClO.
Arrange the following oxyacids of chlorine in decreasing order of their thermal stability. Give reason.
HOClO, HOCl, HClO4, HOClO2
The increasing order of reducing power of the halogen acids is:
Which one of the following order is correct for the bond dissociation enthalpy of halogen molecule?
Assertion: HI cannot be prepared by the reaction of KI with concentrated H2SO4.
Reason: HI has the lowest H-X bond strength among halogen acids.
A black compound of manganese reacts with a halogen acid to give greenish-yellow gas. When excess of this gas reacts with \[\ce{NH3}\] an unstable trihalide is formed. In this process the oxidation state of nitrogen changes from ______.
Explain why the stability of oxoacids of chlorine increases in the order given below:
\[\ce{HClO < HClO2 < HClO3 < HClO4}\]
Assertion: \[\ce{HI}\] cannot be prepared by the reaction of KI with concentrated \[\ce{H2SO4}\]
Reason: \[\ce{HI}\] has lowest \[\ce{H - X}\] bond strength among halogen acids.
Which halogen forms an oxyacid that contains the halogen atom in tripositive oxidation state?
Which among the following factors is the most important in making fluorine, the strongest oxidizing halogen?
