HSC Science (General)
HSC Science (Electronics)
HSC Science (Computer Science)
Academic Year: 2024-2025
Date: March 2025
Advertisements
General Instructions: The question paper is divided into four sections.
- Section A: Q.No.1 contains Ten multiple-choice types of questions carrying One mark each. Q.No.2 contains Eight very short answer type of questions carrying One mark each.
- Section B: Q.No.3 to Q.No.14 are Twelve short answer type questions carrying Two marks each. (Attempt any Eight).
- Section C: Q.No.15 to Q.No.26 are Twelve short answer type questions carrying Three marks each. (Attempt any Eight).
- Section D: Q.No.27 to Q.No.31 are Five Long answer-type questions carrying Four marks each. (Attempt any Three).
- Use of Log table is allowed. Use of calculator is not allowed.
- Figures to the right indicate full marks.
- For each multiple-choice type question, it is mandatory to write the correct answer along with its alphabet. e.g., (a) .............. /(b) ............... /(c) ............... /(d) ................ ,etc. No mark(s) shall be given if ONLY the correct answer or the alphabet of the correct answer is written.
Only the first attempt will be considered for evaluation.
Choose the most correct option.
Components of Nichrome alloy are _______.
Ni, Cr, Fe
Ni, Cr, Fe, C
Ni, Cr
Cu, Fe
Chapter: [0.08] Transition and Inner Transition Elements
Choose the most correct option.
Pressure cooker reduces cooking time for food because _______.
boiling point of water involved in cooking is increased
heat is more evenly distributed in the cooking space
the higher pressure inside the cooker crushes the food material
cooking involves chemical changes helped by a rise temperature
Chapter: [0.02] Solutions
Choose the most correct option.
A living cell contains a solution which is isotonic with 0.3 M sugar solution. What osmotic pressure develops when the cell is placed in 0.1 M KCl solution at body temperature?
5.08 atm
2.54 atm
4.92 atm
2.46 atm
Chapter: [0.02] Solutions [0.02] Solutions and Colligative Properties
Choose the most correct option.
Which of the following is not correct?
Gibbs energy is an extensive property
Electrode potential or cell potential is an intensive property
Electrical work = – ΔG
If half-reaction is multiplied by a numerical factor, the corresponding E° value is also multiplied by the same factor.
Chapter: [0.05] Electrochemistry
Choose the correct option from the given alternatives.
The number of carbon atoms present in the ring of ɛ-caprolactam is ______.
Five
Two
Seven
Six
Chapter: [0.15] Introduction to Polymer Chemistry
In which reaction mechanism carbocation is formed?
SN1
SN2
Both SN1 and SN2
None of them
Chapter: [10.01] Haloalkanes
Select appropriate answers for the following.
Which of the following has highest electron gain enthalpy?
Fluorine
Chlorine
Bromine
Iodine
Chapter: [0.07] Elements of Groups 16, 17 and 18
Isobutylamine is an example of ______.
2° amine
3° amine
1° amine
quaternary ammonium salt
Chapter: [0.13] Amines [13.01] Amines
The molecule of glucose is also called ______.
Glucopyranose
Pyranose
Rabinose
None of them
Chapter: [0.14] Biomolecules
Choose the most correct answer :
For pH > 7 the hydronium ion concentration would be _________.
10-7M
< 10-7M
> 10-7M
≥ 10-7M
Chapter: [0.03] Ionic Equilibria
Answer the following in one sentence.
Write examples of addition polymers and condensation polymers.
Chapter: [0.15] Introduction to Polymer Chemistry
Why tryptophan is an essential amino acid?
Chapter: [14.02] Proteins
Answer in one sentence.
Which amide does produce ethanamine by Hofmann bromamide degradation reaction?
Chapter: [0.13] Amines [13.01] Amines
Answer in one sentence/ word.
Write the name of the electrophile used in Kolbe’s Reaction.
Chapter: [0.11] Alcohols, Phenols and Ethers
Answer the following in one or two sentences.
What is the relationship between coefficients of reactants in a balanced equation for an overall reaction and exponents in the rate law? In what case the coefficients are the exponents?
Chapter: [0.06] Chemical Kinetics
Answer in one sentence.
Write the reaction of p-toluenesulphonyl chloride with diethylamine.
Chapter: [0.13] Amines
Answer the following in brief.
How instantaneous rate of reaction is determined?
Chapter: [0.06] Chemical Kinetics
Advertisements
Name a compound where Frenkel defect is found.
Chapter: [0.01] Solid State
Why alkyl halides though polar are immiscible with water?
Chapter: [10.01] Haloalkanes
Answer in brief.
What are ionization isomers ? Give an example.
Chapter: [0.09] Coordination Compounds
Answer the following.
What are isotonic and hypertonic solutions?
Chapter: [0.02] Solutions [0.02] Solutions and Colligative Properties
Give scientific reasons:
α-Amino acids have high melting points compared to the corresponding amines or carboxylic acids of comparable molecular mass.
Chapter: [0.14] Biomolecules
Write IUPAC names of following compounds.
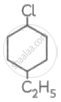
Chapter: [0.1] Halogen Derivatives
Write IUPAC names of following compounds.
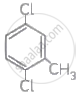
Chapter: [0.1] Halogen Derivatives
Answer the following in brief.
Construct a galvanic cell from the electrodes Co3+ | Co and Mn2+/Mn. `"E"_"Co"^circ` = 1.82 V, `"E"_"Mn"^circ` = –1.18 V. Calculate `"E"_"cell"^circ`
Chapter: [0.05] Electrochemistry
Answer the following in one or two sentences.
Write Nernst equation. What part of it represents the correction factor for nonstandard state conditions?
Chapter: [0.05] Electrochemistry
Give the reactions involved in the Etard's reaction.
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids [12.01] Aldehydes and Ketones
Answer the following in brief :
What are acids and bases according to Arrhenius theory?
Chapter: [0.03] Ionic Equilibria
Give reason:
Alkyl halides are generally not prepared by free radical halogenation of alkanes.
Chapter: [0.1] Halogen Derivatives
Answer the following
Give the full form (long form) of the name for following instrument.
XRD
Chapter: [0.16] Green Chemistry and Nanochemistry
Answer the following
Give the full form (long form) of the name for the following instrument.
TEM
Chapter: [0.16] Green Chemistry and Nanochemistry
Complete the following reaction sequence by writing the structural formulae of the organic compound 'A', 'B' and 'C'.
\[\ce{2-Bromobutane->[Alc.KOH]A->[][Br2]B->[][NaNH2]C}\]
Chapter: [0.1] Halogen Derivatives
The vapour pressure of water at 20°C is 17 mm Hg. What is the vapour pressure of solution containing 2.8 g urea in 50 g of water?
Chapter: [0.02] Solutions
What are pseudo-first-order reactions?
Chapter: [0.06] Chemical Kinetics
Answer the following in brief.
Give one example and explain why it is pseudo-first-order.
Chapter: [0.06] Chemical Kinetics
Attempt the following:
Write preparation, properties and uses of Teflon.
Chapter: [0.15] Introduction to Polymer Chemistry [0.15] Polymers
Answer the following question.
Give valence bond description for the bonding in the complex [VCl4]-. Draw box diagrams for the free metal ion. Which hybrid orbitals are used by the metal? State the number of unpaired electrons.
Chapter: [0.09] Coordination Compounds
Distinguish between ethylamine, diethylamine, and triethylamine by using Hinsberg’s reagent?
Chapter: [0.13] Amines
Advertisements
Calculate the standard enthalpy of:
\[\ce{N2H_{4(g)} + H_{2(g)} -> 2NH_{3(g)}}\]
If ΔH0(N – H) = 389 kJ mol–1, ΔH0(H – H) = 435 kJ mol–1, ΔH0(N – N) = 159 kJ mol–1.
Chapter: [0.04] Chemical Thermodynamics
The enthalpy change of the following reaction:
\[\ce{CH_{4(g)} + Cl_{2(g)} -> CH3Cl_{(g)} + HCl_{(g)}ΔH^0 = –104 kJ}\]
Calculate C – Cl bond enthalpy. The bond enthalpies are:
| Bond | C − H | Cl − Cl | H − Cl |
| ∆H°/kJ mol−1 | 414 | 243 | 431 |
Chapter: [0.04] Chemical Thermodynamics
Write a note on Stephen reaction.
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids
Write reaction showing aldol condensation of cyclohexanone.
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids
How acetone is converted into propane.
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids
How propanal is converted into propane.
Chapter: [0.12] Aldehydes, Ketones and Carboxylic Acids
Aluminium crystallises in a cubic close-packed structure with a unit cell edge length of 353.6 pm. What is the radius of Al atom? How many unit cells are there in 1.00 cm3 of Al?
Chapter: [0.01] Solid State
Answer the following
What is meant by diamagnetic and paramagnetic? Give one example of diamagnetic and paramagnetic transition metal and lanthanoid metal.
Chapter: [0.08] Transition and Inner Transition Elements
Answer the following question.
State Hess’s law of constant heat summation. Illustrate with an example. State its applications.
Chapter: [0.04] Chemical Thermodynamics
Give the properties of Lanthanoids.
Chapter: [0.08] Transition and Inner Transition Elements
Answer the following.
Give two reactions showing the oxidising property of concentrated H2SO4.
Chapter: [0.07] Elements of Groups 16, 17 and 18
Draw the structures of the HClO3.
Chapter: [7.03] Group 17 Elements
Write the structure of HClO4.
Chapter: [0.07] Elements of Groups 16, 17 and 18
Describe the manufacturing of H2SO4 by the contact process.
Chapter: [0.07] Elements of Groups 16, 17 and 18
The density of iridium is 22.4 g/cm3. The unit cell of iridium is fcc. Calculate the radius of iridium atom. Molar mass of iridium is 192.2 g/mol.
Chapter: [0.01] Solid State
Explain optical isomerism in 2-chlorobutane.
Chapter: [0.1] Halogen Derivatives
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
Maharashtra State Board previous year question papers 12th Standard Board Exam Chemistry with solutions 2024 - 2025
Previous year Question paper for Maharashtra State Board 12th Standard Board Exam Chemistry-2025 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of Maharashtra State Board 12th Standard Board Exam.
How Maharashtra State Board 12th Standard Board Exam Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
