Advertisements
Advertisements
Question
Draw two possible isomers of the compound with molecular formula C3H6O and write their names.
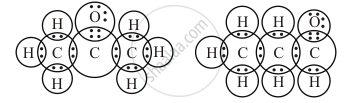
Give the electron dot structures of the above two compounds.
Solution
Two possible isomers of the compound with molecular formula C3H6O:
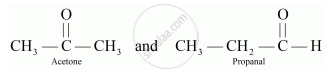
Electron dot structure:
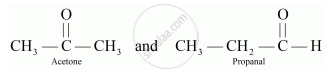
APPEARS IN
RELATED QUESTIONS
Write the number of covalent bonds in the molecule of propane, C3H8.
Draw the structures for Bromopentane*
Draw the structures for Butanone
Draw the structures for Hexanal.
Why does the element carbon from a large number of carbon compounds?
You are given the following molecular formulae of some hydrocarbons:
Which formula represents benzene?
You are given the following molecular formulae of some hydrocarbons:
C5H8; C7H14; C6H6; C5H10; C7H12; C6H12
Which three formulae represent open chain unsaturated hydrocarbons having double bonds?
You are given the following molecular formulae of some hydrocarbons:
C5H8; C7H14; C6H6; C5H10; C7H12; C6H12
Which three formulae can represent cyclic hydrocarbons?
Write the molecular and structural formula of a cyclic hydrocarbon whose molecule contains 8 atoms of carbon.
Recognise the carbon chain type of the following:
\[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\\\ce{H - C - C - C - C - H}\\|\phantom{....}|\phantom{....}|\phantom{....}|\\
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}
\end{array}\]
