Advertisements
Advertisements
Question
Explain the following terms with suitable examples : Interstitials
Solution
Interstitials: Interstitial defect is shown by non-ionic solids. This type of defect is created when some constituent particles (atoms or molecules) occupy an interstitial site of the crystal. The density of a substance increases because of this defect.
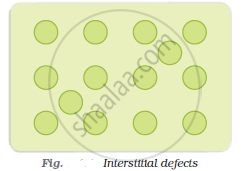
APPEARS IN
RELATED QUESTIONS
Give reasons : In stoichiometric defects, NaCl exhibits Schottky defect and not Frenkel defect.
Examine the given defective crystal:

Answer the following questions :
(i) What type of stoichiometric defect is shown by the crystal?
(ii) How is the density of the crystal affected by this defect?
(iii) What type of ionic substances show such defect?
Answer the following question.
What type of stoichiometric defect is shown by ZnS and why?
Defects in solids can be studied using
Point defect is also known as ____________.
Dislocation defect is also known as ____________.
Frenkel defects are not found in alkali metal halides because ____________.
Type of stoichiometric defect shown by ZnS is ____________.
The following diagram shows:
Silver halides generally show:
What is the effect of Frenkel defect on the density of ionic solids?
Cations are present in the interstitial sites in ______.
Which of the following statements are not true?
(i) Vacancy defect results in a decrease in the density of the substance.
(ii) Interstitial defects results in an increase in the density of the substance.
(iii) Impurity defect has no effect on the density of the substance.
(iv) Frankel defect results in an increase in the density of the substance.
Which of the following defects decrease the density?
(i) Interstitial defect
(ii) Vacancy defect
(iii) Frankel defect
(iv) Schottky defect
Given below are two statements, one is labelled as Assertion (A) and the other is labelled as Reason (R):
Assertion: In any ionic solid (MX) with Schottky defects, the number of positive and negative ions are same.
Reason: Equal number of cation and anion vacancies are present.
Given below are two statements:
Statements I: Frenkel defects are vacancy as well as interstitial defects.
Statements II: Frenkel defect leads of colour in ionic solids due to the presence of F-centers.
Choose the most appropriate answer for the statements from the options given below:
The incorrect statement about the imperfections in solids is ______.
Which type of 'defect' has the presence of cations in the interstitial sites?
