Advertisements
Advertisements
Question
Explain in brief with one example of an ionic bond.
Solution
The bond formed by the complete transfer of one or more electrons from an electropositive atom to an electronegative atom, leading to the formation of ions that are held together by electrostatic attraction is called an ionic bond or electrovalent bond.
Formation of sodium chloride (NaCl):
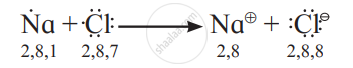
- The electronic configurations of sodium and chlorine are:
Na (Z = 11): 1s2 2s2 2p6 3s1 or (2, 8, 1)
Cl (Z = 17): 1s2 2s2 2p6 3s2 3p5 or (2, 8, 7) - Sodium has one electron in its valence shell. It has a tendency to lose one electron to acquire the electronic configuration of the nearest inert gas, neon (2, 8).
- Chlorine has seven electrons in its valence shell. It has a tendency to gain one electron and thereby acquire the electronic configuration of the nearest inert gas, argon (2, 8, 8).
- During the combination of sodium and chlorine atoms, the sodium atom transfers its valence electron to the chlorine atom.
- Sodium atom changes into Na+ ion while the chlorine atom changes into Cl ion. The two ions are held together by strong electrostatic force of attraction.
- The formation of ionic bond between Na and Cl can be shown as follows:
\[\ce{Na^+ + Cl^-->\underset{\text{Ionic bond}}{Na^+Cl^-}}\] or NaCl
APPEARS IN
RELATED QUESTIONS
Write Lewis symbols for the following atoms and ions: S and S2–.
Write Lewis symbols for the following atoms and ions: Al and Al3+.
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions:-
K and S
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions:
Al and N
Select and write the most appropriate alternatives from the given choices.
When the following bond types are listed in decreasing order of strength (strongest first). Which is the correct order?
Draw Lewis dot diagram for the following.
Hydrogen (H2)
Draw Lewis dot diagram for the following.
Carbon dioxide (CO2)
Draw Lewis dot diagram for the following.
Methane (CH4)
Draw Lewis dot diagram for the following.
Lifthium Fluoride (LiF)
Draw Lewis electron dot structure of HF
Draw Lewis electron dot structure of C2H6
Draw Lewis electron dot structure of CF3Cl
Draw Lewis electron dot structure of SO2
Which of the following molecules has a central atom with complete octet?
____________ is an example of molecule with expanded octet.
In which of the following, the central atom does NOT have lone pair(s) of electrons?
The condensed electronic configuration [Ne] 3s2 3p5 denotes the element ____________.
Which of the following molecule does not obey octet rule?
Which of the following molecule does not obey octet rule?
Assertion (A): Sodium chloride formed by the action of chlorine gas on sodium metal is a stable compound.
Reason (R): This is because sodium and chloride ions acquire octet in sodium chloride formation.
