Advertisements
Advertisements
Question
Write Lewis symbols for the following atoms and ions: Al and Al3+.
Solution 1
Al and Al3+
The number of valence electrons in aluminium is 3.
The Lewis dot symbol of aluminium (Al) is 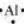 .
.
The tripositive charge on a species infers that it has donated its three electrons. Hence, the Lewis dot symbol is 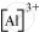
Solution 2
`""_13"Al"` = 2,8,3 ∴ Lewis symbol = 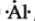 , `"Al"^(3+) "ions" = ["Al"]^(3+)`.
, `"Al"^(3+) "ions" = ["Al"]^(3+)`.
APPEARS IN
RELATED QUESTIONS
Explain the Formation of a Chemical Bond.
Write Lewis symbols for the following atoms and ions: S and S2–.
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions:
Ca and O
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions:
Al and N
Draw Lewis dot diagram for the following.
Water (H2O)
Draw Lewis dot diagram for the following.
Carbon dioxide (CO2)
Draw Lewis dot diagram for the following.
Methane (CH4)
Draw Lewis electron dot structure of C2H6
Draw Lewis electron dot structure of C2H4
Draw Lewis electron dot structure of SO2
Explain in brief with one example of an ionic bond.
Which of the following molecules has a central atom with complete octet?
Which of the following is INCORRECT with respect to enthalpy?
In which of the following, the central atom does NOT have lone pair(s) of electrons?
The condensed electronic configuration [Ne] 3s2 3p5 denotes the element ____________.
Which of the following molecule does not obey octet rule?
What is the formal charge on hydrogen atom in water molecule?
Which of the following molecule does not obey octet rule?
