Advertisements
Advertisements
Question
Explain the following term:
Substitutional impurity defect
Solution
Substitutional impurity defect: In this defect, the foreign atoms are found at the lattice sites in place of host atoms. The regular atoms are displaced from their lattice sites by impurity atoms.
For example :
- Solid solutions of metals (alloys): Brass is an alloy of Cu and Zn. In brass, host Cu atoms are replaced by the impurity of Zn atoms. The Zn atoms occupy regular sites of Cu atoms as shown in the figure.
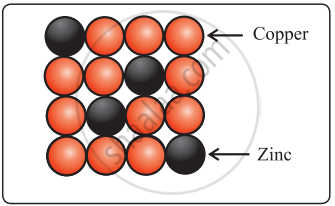 Brass Brass |
APPEARS IN
RELATED QUESTIONS
What are the consequences of Schottky defect?
Explain with diagram, the vacancy defect.
How are nonstoichiometric point defects classified? Explain with diagram the metal deficiency defect.
What is a substitutional impurity defect?
Explain solid solutions of metals and vacancy through aliovalent cations.
Which of the following is an example of substitutional impurity defect?
When a cation or anion from ionic solid leaves its regular lattice site and moves to occupy an interstitial site, it is called as ____________.
Which among the following solids shows Frenkel defect?
Which among the following statements is true about Schottky defect?
Which among the following defects is observed in Brass?
Schottky defect is formed in ionic compounds with ____________.
Write the consequences of Schottky defect with reasons.
Explain metal deficiency defect with examples.
AgBr shows which type of defect?
Name a compound where Frenkel defect is found.
In which among the following solids, Schottky defect is not observed?
Explain the following term:
Interstitial impurity defect
Explain Self interstitial defect in elemental solid.
Explain the interstitial defect.
What is a vacancy defect?
What are point defects?
