Advertisements
Advertisements
Question
Explain, why solid sodium chloride does not allow electricity to pass through?
Solution
In sodium chloride, Na+ and Cl- ions are not free to carry the electric current.
APPEARS IN
RELATED QUESTIONS
State the observations at the anode and at the cathode during the electrolysis of copper sulphate solution using copper electrodes.
Complete the following by selecting the correct option from the choices given:
The divalent metal whose oxide is reduced to metal by electrolysis of its fused salt is __________. (Al/Na/Mg/K)
Name the product formed at the anode during the electrolysis of acidified water using platinum electrodes.
Copy and complete the following table which refers to the conversion of ions to neutral particles.
| Conversion | Ionic equation | Oxidation / Reduction |
| Chloride ion to chlorine molecule | 1)_________ | 2)_________ |
| Lead (II) ion to lead | 3)_________ | 4)_________ |
Define the term : Electrolyte
What kind of particles will be found in a liquid compound which is a non- electrolyte?
Explain how electrolysis is an example of Redox reaction.
Here is an electrode reaction:
\[\ce{Cu -> Cu^{2+} + 2e^-}\]
At which electrode (anode or cathode) would such a reaction take place? Is this an example of oxidation?
Copper sulphate solution is electrolyzed using copper electrodes. Study the diagram given alongside and answer the questions that follow.
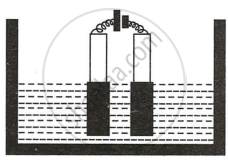
- Which electrode to your left or right is known as the oxidizing electrode and why?
- Write the equation representing the reaction that occurs.
- State two appropriate observations for the above electrolysis reactions.
From the following list of substances choose those which meet the description given below.
Ammonium chloride, ammonium nitrate, chlorine, dilute hydrochloric acid, iron, lead nitrate, manganese (IV) oxide, silver nitrate, sodium nitrate, sodium nitrite, and sulphur.
Two compounds whose aqueous solutions give white precipitates with dilute hydrochloric acid.
