Advertisements
Advertisements
Question
Fill in the blank
Most electronegative elements belong to ______ group.
Solution
Most electronegative elements belong to Seventeen group.
APPEARS IN
RELATED QUESTIONS
Choose the most appropriate answer of the following:
Among the elements given below, the element with the least electronegativity is:
(A) Lithium
(B) Carbon
(C) Boron
(D) Fluorine
State the character of the oxide of period 3 ?
Study the extract of the Periodic Table given below and answer the questions that follow. Give the alphabet corresponding to the element in question. DO NOT repeat an element. 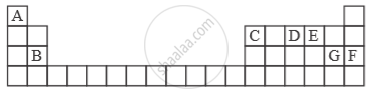
Which element forms electrovalent compound with G?
In terms of electronic configuration, what the elements of a given period and groups have in common?
What property of an element is measured by electro negativity?
If the electronegativity difference between two bonded atoms in a molecule is greater than 1.7, the nature of bonding is ______
Answer the following question using the data given below:
- Assertion: The nature of bond in HF molecule is ionic
- Reason: The electronegativity difference between H and F is 1.9.
Identify the following:
The most electronegative element of Period 3.
Electronegativity across the period ______.
