Advertisements
Advertisements
Question
Fill in the blank
The maximum electro negativity is shown by ______.
Solution
The maximum electro negativity is shown by Fluorine.
APPEARS IN
RELATED QUESTIONS
Rewrite the following sentences by using the correct symbol > (greater than) or < (less than) in the blanks given
The electronegativity of iodine is ___________ that of Chlorine
Define the term Electronegativity.
State the character of the oxide of period 3 ?
Study the extract of the Periodic Table given below and answer the questions that follow. Give the alphabet corresponding to the element in question. DO NOT repeat an element. 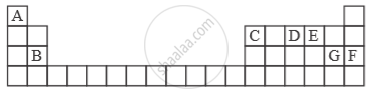
Which element forms electrovalent compound with G?
Fill in the blank
Most electronegative elements belong to ______ group.
Select the correct answer
Which of the following element has highest electronegativity?
The electro negativities (according to Pauling) of the elements in period 3 of the periodic table are as follows.
The elements are arranged in alphabetical order:
Al Cl Mg Na P S Si
1.5 3.0 1.2 0.9 2.1 2.5 1.8
(i) Arrange the elements in the order in which they occur in the periodic table from left to right.
(The group 1 elements first, followed by the group 2 element and so on, up to group 7)
(ii) Choose the word or phrase from the brackets which correctly completes each of the following statements:
(a) The element below sodium in the same group would be expected to have a ______ (lower/higher) electro negativity than sodium and the element above chlorine which would be expected to have a ______ (lower / higher) ionization potential than chlorine.
(b) On moving from left to right in a given period, the number of shells ______ (remains the same / decreases/ increases).
(c) On moving down a group, the number of valence electrons ______ (remains the same / increases/ decreases).
What property of an element is measured by electro negativity?
The element below sodium in the same group would be expected to have a ______ (lower/higher) electro-negativity than sodium and the element above chlorine would be expected to have a ______ (lower/higher) ionization potential than chlorine.
