Advertisements
Advertisements
Questions
Fill in the blanks using the appropriate words given below :
(Sulphur dioxide, nitrogen dioxide, Nitric oxide, Sulphuric acid)
Cold, dilute nitric acid reacts with copper to give ___________.
Fill in the blanks from the choices given within brackets:
Cold, dilute nitric acid reacts with copper to form_______ (hydrogen, nitrogen dioxide, nitric oxide).
Solution
Nitric oxide
RELATED QUESTIONS
What is aqua fortis?
What is fixation of Nitrogen?
Nitric acid cannot be concentrated beyond 68% by the distillation of a dilute solution of \[\ce{HNO3}\]. State the reason.
Write a balanced equation and name the products formed when sodium hydrogen carbonate is added to nitric acid.
Write down the word equation or balanced equation for the action of concentrated nitric acid on copper.
Write equation to show the reaction between the following:
Copper and concentrated nitric acid.
Write balanced equation to show the reaction between the sulphur and hot concentrated nitric acid.
Fill in the blank using the appropriate words given below:
Hot, concentrated nitric add reacts with sulphur to form ______.
Write a balanced equation for following :
Action of cold and dilute nitric acid on copper
The diagram given below is a representation of the Industrial preparation of Nitric acid by Ostwald’s process. With respect to the process answer the following questions:
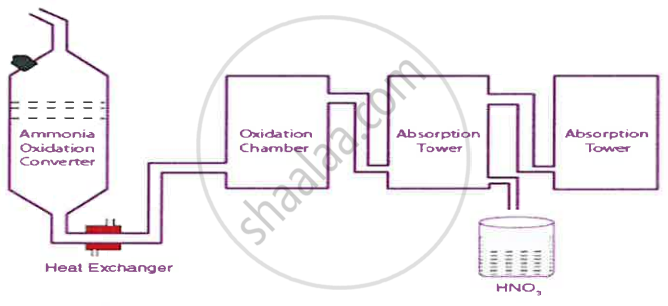
- Write the temperature and the catalyst required during the catalytic oxidation of ammonia.
- Give balanced chemical equation for the reaction occurring duringthe conversion of nitrogen dioxide to nitric acid.
