Advertisements
Advertisements
Question
Find the angular momentum of an electron revolving in the second orbit in Bohr's hydrogen atom.
Solution
The angular momentum of an electron revolving around the nucleus in a hydrogen atom is quantised and given by
`L = (nh)/(2pi)` For n = 2
`L = (2h)/(2pi) = h/pi = (6.6 xx 10^-34)/3.14`
= `2.10 xx 10^34` kg m2s-1
APPEARS IN
RELATED QUESTIONS
(i) State Bohr's quantization condition for defining stationary orbits. How does the de Broglie hypothesis explain the stationary orbits?
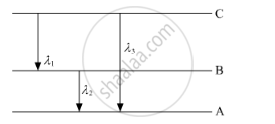
(ii) Find the relation between three wavelengths λ1, λ2 and λ3 from the energy-level diagram shown below.
Calculate the radius of second Bohr orbit in hydrogen atom from the given data.
Mass of electron = 9.1 x 10-31kg
Charge on the electron = 1.6 x 10-19 C
Planck’s constant = 6.63 x 10-34 J-s.
Permittivity of free space = 8.85 x 10-12 C2/Nm2
Calculate the energy required for the process
\[\ce{He^+_{(g)} -> He^{2+}_{(g)} + e^-}\]
The ionization energy for the H atom in the ground state is 2.18 ×10–18 J atom–1
If the photon of the wavelength 150 pm strikes an atom and one of its inner bound electrons is ejected out with a velocity of 1.5 × 107 ms–1, calculate the energy with which it is bound to the nucleus.
Using Bohr's postulates, derive the expression for the total energy of the electron in the stationary states of the hydrogen atom ?
The electron in hydrogen atom is initially in the third excited state. What is the maximum number of spectral lines which can be emitted when it finally moves to the ground state?
The light emitted in the transition n = 3 to n = 2 in hydrogen is called Hα light. Find the maximum work function a metal can have so that Hα light can emit photoelectrons from it.
The ratio of the ionization energy of H and Be+3 is ______.
Given below are two statements:
Statements I: According to Bohr's model of an atom, qualitatively the magnitude of velocity of electron increases with decrease in positive charges on the nucleus as there is no strong hold on the electron by the nucleus.
Statement II: According to Bohr's model of an atom, qualitatively the magnitude of velocity of electron increase with a decrease in principal quantum number.
In light of the above statements, choose the most appropriate answer from the options given below:
Find the ratio of energies of photons produced due to transition of an election of hydrogen atom from its (i) second permitted energy level to the first level. and (ii) the highest permitted energy level to the first permitted level.
