Advertisements
Advertisements
Question
For a given reaction at a particular temperature, the equilibrium constant has a constant value. Is the value of Q also constant? Explain.
Solution
Kc and Qc are constant at equilibrium, both are temperature dependent. When Kc is constant at a given temperature, Qc also constant.
APPEARS IN
RELATED QUESTIONS
Which one of the following is incorrect statement ?
K1 and K2 are the equilibrium constants for the reactions respectively.
\[\ce{N2(g) + O2(g) <=>[K1] 2NO(g)}\]
\[\ce{NO(g) + O2(g) <=>[K2] 2NO2(g)}\]
What is the equilibrium constant for the reaction \[\ce{NO2(g) <=> 1/2 N2(g) + O2(g)}\]
In the equilibrium,
\[\ce{2A(g) <=> 2B(g) + C2(g)}\]
the equilibrium concentrations of A, B and C2 at 400 K are 1 × 10–4 M, 2.0 × 10–3 M, 1.5 × 10–4 M respectively. The value of KC for the equilibrium at 400 K is
For the reaction \[\ce{AB(g) <=> A(g) + B(g)}\], at equilibrium, AB is 20 % dissociated at a total pressure of P, the equilibrium constant Kp is related to the total pressure by the expression
In which of the following equilibrium, Kp and Kc are not equal?
In a chemical equilibrium, the rate constant for the forward reaction is 2.5 × 10-2, and the equilibrium constant is 50. The rate constant for the reverse reaction is,
For the reaction, \[\ce{A2(g) + B2(g) <=> 2AB(g); \Delta H}\] is -ve.
the following molecular scenes represent differenr reaction mixture. (A-green, B-blue)
| Closed ← |
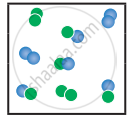 |
 |
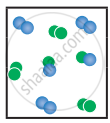 |
| System | At equilibrium | (x) | (y) |
- Calculate the equilibrium constant Kp and (Kc).
- For the reaction mixture represented by scene (x), (y) the reaction proceed in which directions?
- What is the effect of an increase in pressure for the mixture at equilibrium?
Write the balanced chemical equation for an equilibrium reaction for which the equilibrium constant is given by expression.
`"K"_"C" = (["NH"_3]^4["O"_2]^5)/(["NO"]^4["H"_2"O"]^6)`
For the reaction
\[\ce{SrCO3(s) <=> SrO(s) + CO2(g)}\]
the value of equilibrium constant Kp = 2.2 × 10-4 at 1002 K. Calculate Kc for the reaction.
To study the decomposition of hydrogen iodide, a student fills an evacuated 3 litre flask with 0.3 mol of HI gas and allows the reaction to proceed at 500°C. At equilibrium he found the concentration of HI which is equal to 0.05 M. Calculate Kc and Kp.
