Advertisements
Online Mock Tests
Chapters
2: Quantum Mechanical Model of Atom
3: Periodic Classification Of Elements
4: Hydrogen
5: Alkali and Alkaline Earth Metals
6: Gaseous State
7: Thermodynamics
▶ 8: Physical and Chemical Equilibrium
9: Solutions
10: Chemical bonding
11: Fundamentals of Organic Chemistry
12: Basic concept of organic reactions
13: Hydrocarbons
14: Haloalkanes and Haloarenes
15: Environmental Chemistry
![Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 11 TN Board chapter 8 - Physical and Chemical Equilibrium Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 11 TN Board chapter 8 - Physical and Chemical Equilibrium - Shaalaa.com](/images/chemistry-volume-1-and-2-english-class-11-tn-board_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
Advertisements
Solutions for Chapter 8: Physical and Chemical Equilibrium
Below listed, you can find solutions for Chapter 8 of Tamil Nadu Board of Secondary Education Samacheer Kalvi for Chemistry - Volume 1 and 2 [English] Class 11 TN Board.
Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 11 TN Board 8 Physical and Chemical Equilibrium Evaluation [Pages 22 - 28]
Choose the best answer
If Kb and Kf for a reversible reaction are 0.8 × 10-5 and 1.6 × 10-4 respectively, the value of the equilibrium constant is,
20
0.2 × 10-1
0.05
None of these
At a given temperature and pressure, the equilibrium constant values for the equilibria
\[\ce{3A2 + B2 + 2Cr <=>[K1] 2A3BC}\] and
\[\ce{A3BC <=> 3/2 A2 + 1/2 B2 + C}\]
The relation between K1 and K2 is
`"K"_1 = 1/sqrt"K"_2`
`"K"_2 = "K"_1^(-1//2)`
`"K"_1^2 = 2"K"_2`
`"K"_1/2 = "K"_2`
The equilibrium constant for a reaction at room temperature is K1 and that at 700 K is K2 If K1 > K2, then
The forward reaction is exothermic
The forward reaction is endothermic
The reaction does not attain equilibrium
The reverse reaction is exothermic
The formation of ammonia from N2(g) and H2(g) is a reversible reaction
\[\ce{N2(g) + 3H2(g) <=> 2NH3(g) + Heat}\]
What is the effect of increase of temperature on this equilibrium reaction.
equilibrium is unaltered
formation of ammonia is favoured
equilibrium is shifted to the left
reaction rate does not change
Solubility of carbon dioxide gas in cold water can be increased by ______.
increase in pressure
decrease in pressure
increase in volume
none of these
Which one of the following is incorrect statement ?
for a system at equilibrium, Q is always less than the equilibrium constant
equilibrium can be attained from either side of the reaction
presence of catalyst affects both the forward reaction and reverse reaction to the same extent
Equilibrium constant varied with temperature
K1 and K2 are the equilibrium constants for the reactions respectively.
\[\ce{N2(g) + O2(g) <=>[K1] 2NO(g)}\]
\[\ce{NO(g) + O2(g) <=>[K2] 2NO2(g)}\]
What is the equilibrium constant for the reaction \[\ce{NO2(g) <=> 1/2 N2(g) + O2(g)}\]
`1/(sqrt("K"_1 "K"_2))`
(K1 = K2)1/2
`1/(2"K"_1"K"_2)`
`(1/("K"_1"K"_2))^(3//2)`
In the equilibrium,
\[\ce{2A(g) <=> 2B(g) + C2(g)}\]
the equilibrium concentrations of A, B and C2 at 400 K are 1 × 10–4 M, 2.0 × 10–3 M, 1.5 × 10–4 M respectively. The value of KC for the equilibrium at 400 K is
0.06
0.09
0.62
3 × 10-2
An equilibrium constant of 3.2 × 10-6 for a reaction means, the equilibrium is
largely towards forward direction
largely towards reverse direction
never established
none of these
`"K"_"C"/"K"_"P"` for the reaction,
\[\ce{N2(g) + 3H2(g) <=> 2NH3(g)}\] is
`1/"RT"`
`sqrt"RT"`
RT
(RT)2
For the reaction \[\ce{AB(g) <=> A(g) + B(g)}\], at equilibrium, AB is 20 % dissociated at a total pressure of P, the equilibrium constant Kp is related to the total pressure by the expression
P = 24 Kp
P = 8 Kp
24 P = Kp
none of these
In which of the following equilibrium, Kp and Kc are not equal?
\[\ce{2NO(g) <=> N2(g) + O2(g)}\]
\[\ce{SO2(g) + NO2 <=> SP3(g) + NO(g)}\]
\[\ce{H2(g) + I2(g) <=> 2HI(g)}\]
\[\ce{PCl5(g) <=> PCl3(g) + Cl2 (g)}\]
If x is the fraction of PCl5 dissociated at equilibrium in the reaction
\[\ce{PCl5 <=> PCl3 + Cl2}\]
then starting with 0.5 mole of PCl5, the total number of moles of reactants and products at equilibrium is
0.5 – x
x + 0.5
2x + 0.5
x + 1
The values of Kp1 and Kp2; for the reactions,
X ⇌ Y + Z,
A ⇌ 2B are in the ratio 9 : 1 if degree of dissociation of X and A be equal then total pressure at equilibrium P1, and P2 are in the ratio
36 : 1
1 : 1
3 : 1
1 : 9
In the reaction
\[\ce{Fe(OH)3(S) <=> Fe^{(3+)} (aq) + 3OH- (aq)}\]
if the concentration of OH– ions is decreased by 1/4 times, then the equilibrium concentration of Fe3+ will
not changed
also decreased by 1/4 times
increase by 4 times
increase by 64 times
Consider the reaction where Kp = 0.5 at a particular temperature
\[\ce{PCl5(g) <=> PCl3 (g) + Cl2 (g)}\]
if the three gases are mixed in a container so that the partial pressure of each gas is initially 1 atm, then which one of the following is true.
more PCl3 will be produced
more Cl2 will be produced
more PCl5 will be produced
None of these
Equimolar concentrations of H2 and I2 are heated to equilibrium in a 1 liter flask. What percentage of the initial concentration of H2 has reacted at equilibrium if the rate constant for both forward and reverse reactions are equal
33%
66%
(33)2%
16.5%
In a chemical equilibrium, the rate constant for the forward reaction is 2.5 × 10-2, and the equilibrium constant is 50. The rate constant for the reverse reaction is,
11.5
50
2 × 102
2 × 10-3
Which of the following is not a general characteristic of equilibrium involving physical process
Equilibrium is possible only in a closed system at a given temperature
The opposing processes occur at the same rate and there is a dynamic but stable condition
All the physical processes stop at equilibrium
All measurable properties of the system remains constant
For the formation of Two moles of SO3(g) from SO2 and O2, the equilibrium constant is K1. The equilibrium constant for the dissociation of one mole of SO3 into SO2 and O2 is
`1/"K"_1`
`"K"_1^2`
`(1/"K"_1)^(1/2)`
`"K"_1/2`
Match the equilibria with the corresponding conditions:
- Liquid ⇌ Vapour
- Solid ⇌ Liquid
- Solid ⇌ Vapour
- Solute(s) ⇌ Solute (Solution)
- Melting point
- Saturated solution
- Boiling point
- Sublimation point
- Unsaturated solution
(i) - 1, (ii) - 2, (iii) - 3, (iv) - 4
(i) - 3, (ii) - 1, (iii) - 4, (iv) - 2
(i) - 2, (ii) - 1, (iii) - 3, (iv) - 4
(i) - 3, (ii) - 2, (iii) - 4, (iv) - 5
Consider the following reversible reaction at equilibrium,
\[\ce{A + B <=> C}\],
If the concentration of the reactants A and B are doubled, then the equilibrium constant will
be doubled
become one fourth
be halved
remain the same
\[\ce{[CO(H2O)6]^2+ (aq) (pink) + 4Cl- (aq) <=> [CoCl4]^2- (aq) (blue) + 6 H2O (l)}\]
In the above reaction at equilibrium, the reaction mixture is blue in colour at room temperature. On cooling this mixture, it becomes pink in color. On the basis of this information, which one of the following is true?
∆H > 0 for the forward reaction
∆H = 0 for the reverse reaction
∆H < 0 for the forward reaction
Sign of the ∆H cannot be predicted based on this information
The equilibrium constants of the following reactions are:
\[\ce{N2 + 3H2 <=> 2NH3}\]; K1
\[\ce{N2 + O2 <=> 2NO}\]; K2
\[\ce{H2 + 1/2O2 <=> H2O}\]; K3
The equilibrium constant (K) for the reaction;
\[\ce{2NH3 + 5/2 O2 <=> 2NO + 3H2O}\], will be
`"K"_2^3 "K"_3/"K"_1`
`"K"_1 "K"_3^3/"K"_2`
`"K"_2 "K"_3^3/"K"_1`
`"K"_2 "K"_3/"K"_1`
A 20 litre container at 400 K contains CO2 (g) at pressure 0.4 atm and an excess (neglect the volume of solid SrO). The volume of the container is now decreased by moving the movable piston fitted in the container. The maximum volume of the container, when the pressure of CO2 attains its maximum value will be:
Given that: \[\ce{SeCO3(S)≅ SrO + CO2 (g)}\] [Kp = 1.6 atm]
2 litre
5 litre
10 litre
4 litre
Write brief answer to the following questions.
If there is no change in concentration, why is the equilibrium state considered dynamic?
For a given reaction at a particular temperature, the equilibrium constant has a constant value. Is the value of Q also constant? Explain.
What is the relation between Kp and Kc? Given one example for which Kp is equal to Kc.
For a gaseous homogeneous reaction at equilibrium, a number of moles of products is greater than the number of moles of reactants. Is KC is larger or smaller than Kp?
When the numerical value of the reaction quotient (Q) is greater than the equilibrium constant, in which direction does the reaction proceed to reach equilibrium?
For the reaction, \[\ce{A2(g) + B2(g) <=> 2AB(g); \Delta H}\] is -ve.
the following molecular scenes represent differenr reaction mixture. (A-green, B-blue)
| Closed ← |
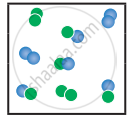 |
 |
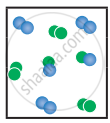 |
| System | At equilibrium | (x) | (y) |
- Calculate the equilibrium constant Kp and (Kc).
- For the reaction mixture represented by scene (x), (y) the reaction proceed in which directions?
- What is the effect of an increase in pressure for the mixture at equilibrium?
State Le – Chateller principle.
Consider the following reaction,
\[\ce{H2(g) + l2(g) <=> 2HI(g)}\]
In the above reactions find out whether you have to increase (or) decrease the volume to increase the yield of the product.
Consider the following reaction,
\[\ce{CaCO3(s) <=> CaO(s) + CO2(g)}\]
In the above reactions find out whether you have to increase (or) decrease the volume to increase the yield of the product.
Consider the following reaction,
\[\ce{S(s) + F2(g) <=> SF6(g)}\]
In the above reactions find out whether you have to increase (or) decrease the volume to increase the yield of the product.
State law of mass action.
Explain how will you predict the direction of an equilibrium reaction.
Derive a general expression for the equilibrium constant Kp and Kc for the reaction, \[\ce{3H2(g) + N2(g) <=> 2NH3(g)}\].
Write the balanced chemical equation for an equilibrium reaction for which the equilibrium constant is given by expression.
`"K"_"C" = (["NH"_3]^4["O"_2]^5)/(["NO"]^4["H"_2"O"]^6)`
What is the effect of added Inert gas on the reaction at equilibrium?
Derive the relation between Kp and Kc.
One mole of PCl5 is heated in one litre closed container. If 0.6 mole of chlorine is found at equilibrium, Calculate the value of equilibrium constant.
For the reaction
\[\ce{SrCO3(s) <=> SrO(s) + CO2(g)}\]
the value of equilibrium constant Kp = 2.2 × 10-4 at 1002 K. Calculate Kc for the reaction.
To study the decomposition of hydrogen iodide, a student fills an evacuated 3 litre flask with 0.3 mol of HI gas and allows the reaction to proceed at 500°C. At equilibrium he found the concentration of HI which is equal to 0.05 M. Calculate Kc and Kp.
1 mol of CH4, 1 mole of CS2 and 2 mol of H2S are 2 mol of H2 are mixed in a 500 ml flask. The equilibrium constant for the reaction Kc = 4 x 10-2 mol2 lit-2. In which direction will the reaction proceed to reach equilibrium?
At particular temperature Kc = 4 × 10-2 for the reaction, \[\ce{H2S (g) <=> H2(g) +1/2 S2(g)}\]. Calculate the Kc for the following reaction.
\[\ce{2H2S (g) <=> 2H2 (g) + S2 (g)}\]
At particular temperature Kc = 4 × 10-2 for the reaction, \[\ce{H2S (g) <=> H2(g) +1/2 S2(g)}\]. Calculate the Kc for the following reaction.
\[\ce{3H2S (g) <=> 3H2 (g) + 3/2 S2 (g)}\]
28 g of Nitrogen and 6 g of hydrogen were mixed In a 1 litre closed container. At equIlibrium 17 g NH3 was produced. Calculate the weight of nitrogen, hydrogen at equilibrium.
The equilibrium for the dissociation of XY2 is given as,
\[\ce{2 XY2 (g) <=> 2 XY (g) + Y2 (g)}\]
if the degree of dissociation x is so small compared to one. Show that 2 Kp = PX3 where P is the total pressure and Kp is the dissociation equilibrium constant of XY2.
A sealed container was filled with 0.3 mol H2(g), 0.4 mol I2(g) and 0.2 mol HI(g) at 800 K and total pressure 1.00 bar. Calculate the amounts of the components in the mixture at equilibrium given that K = 870 for the reaction, \[\ce{A2 (g) + B2 (g) <=> 2 AB (g)}\].
Deduce the Vant Hoff equation.
The equilibrium constant Kp for the reaction \[\ce{N2 (g) + 3H2 (g) <=> 2NH3 (g)}\] is 8.19 × 102 at 298 K and 4.6 × 10-1 at 498 K. Calculate ∆H° for the reaction.
The partial pressure of carbon dioxide in the reaction
\[\ce{CaCO3(s) <=> CaO(s) + CO2(g)}\] is 1.017 × 10-3 atm at 500°C. Calculate Kp at 600°C for the reaction. H for the reaction is 181 KJ mol-1 and does not change in the given range of temperature.
Solutions for 8: Physical and Chemical Equilibrium
![Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 11 TN Board chapter 8 - Physical and Chemical Equilibrium Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 11 TN Board chapter 8 - Physical and Chemical Equilibrium - Shaalaa.com](/images/chemistry-volume-1-and-2-english-class-11-tn-board_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 11 TN Board chapter 8 - Physical and Chemical Equilibrium
Shaalaa.com has the Tamil Nadu Board of Secondary Education Mathematics Chemistry - Volume 1 and 2 [English] Class 11 TN Board Tamil Nadu Board of Secondary Education solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. Samacheer Kalvi solutions for Mathematics Chemistry - Volume 1 and 2 [English] Class 11 TN Board Tamil Nadu Board of Secondary Education 8 (Physical and Chemical Equilibrium) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. Samacheer Kalvi textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Chemistry - Volume 1 and 2 [English] Class 11 TN Board chapter 8 Physical and Chemical Equilibrium are Physical and Chemical Equilibrium, Introduction of Chemical Equilibrium, Homogeneous and Heterogeneous Equilibria, Equilibrium Constants, Applications of Equilibrium Constants, Le-Chatelier's Principle, Van't Hoff Equation.
Using Samacheer Kalvi Chemistry - Volume 1 and 2 [English] Class 11 TN Board solutions Physical and Chemical Equilibrium exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in Samacheer Kalvi Solutions are essential questions that can be asked in the final exam. Maximum Tamil Nadu Board of Secondary Education Chemistry - Volume 1 and 2 [English] Class 11 TN Board students prefer Samacheer Kalvi Textbook Solutions to score more in exams.
Get the free view of Chapter 8, Physical and Chemical Equilibrium Chemistry - Volume 1 and 2 [English] Class 11 TN Board additional questions for Mathematics Chemistry - Volume 1 and 2 [English] Class 11 TN Board Tamil Nadu Board of Secondary Education, and you can use Shaalaa.com to keep it handy for your exam preparation.
