Advertisements
Advertisements
Question
The values of Kp1 and Kp2; for the reactions,
X ⇌ Y + Z,
A ⇌ 2B are in the ratio 9 : 1 if degree of dissociation of X and A be equal then total pressure at equilibrium P1, and P2 are in the ratio
Options
36 : 1
1 : 1
3 : 1
1 : 9
Solution
36 : 1
APPEARS IN
RELATED QUESTIONS
In the equilibrium,
\[\ce{2A(g) <=> 2B(g) + C2(g)}\]
the equilibrium concentrations of A, B and C2 at 400 K are 1 × 10–4 M, 2.0 × 10–3 M, 1.5 × 10–4 M respectively. The value of KC for the equilibrium at 400 K is
An equilibrium constant of 3.2 × 10-6 for a reaction means, the equilibrium is
`"K"_"C"/"K"_"P"` for the reaction,
\[\ce{N2(g) + 3H2(g) <=> 2NH3(g)}\] is
For the formation of Two moles of SO3(g) from SO2 and O2, the equilibrium constant is K1. The equilibrium constant for the dissociation of one mole of SO3 into SO2 and O2 is
For a given reaction at a particular temperature, the equilibrium constant has a constant value. Is the value of Q also constant? Explain.
For the reaction, \[\ce{A2(g) + B2(g) <=> 2AB(g); \Delta H}\] is -ve.
the following molecular scenes represent differenr reaction mixture. (A-green, B-blue)
| Closed ← |
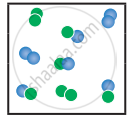 |
 |
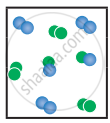 |
| System | At equilibrium | (x) | (y) |
- Calculate the equilibrium constant Kp and (Kc).
- For the reaction mixture represented by scene (x), (y) the reaction proceed in which directions?
- What is the effect of an increase in pressure for the mixture at equilibrium?
For the reaction
\[\ce{SrCO3(s) <=> SrO(s) + CO2(g)}\]
the value of equilibrium constant Kp = 2.2 × 10-4 at 1002 K. Calculate Kc for the reaction.
1 mol of CH4, 1 mole of CS2 and 2 mol of H2S are 2 mol of H2 are mixed in a 500 ml flask. The equilibrium constant for the reaction Kc = 4 x 10-2 mol2 lit-2. In which direction will the reaction proceed to reach equilibrium?
At particular temperature Kc = 4 × 10-2 for the reaction, \[\ce{H2S (g) <=> H2(g) +1/2 S2(g)}\]. Calculate the Kc for the following reaction.
\[\ce{3H2S (g) <=> 3H2 (g) + 3/2 S2 (g)}\]
The partial pressure of carbon dioxide in the reaction
\[\ce{CaCO3(s) <=> CaO(s) + CO2(g)}\] is 1.017 × 10-3 atm at 500°C. Calculate Kp at 600°C for the reaction. H for the reaction is 181 KJ mol-1 and does not change in the given range of temperature.
