Advertisements
Advertisements
Question
Give reason:
Apparatus for laboratory preparation of hydrogen should be airtight and away from a naked flame.
Solution
Apparatus for laboratory preparation of hydrogen should be airtight and away from a naked flame because a mixture of hydrogen and air explodes violently when brought near a flame.
APPEARS IN
RELATED QUESTIONS
Name the following:
A metal which liberates hydrogen only when steam is passed over red hot metal.
Name the following:
A metallic oxide which can be reduced into metal by hydrogen.
Name the chemicals required to prepare hydrogen gas in the laboratory.
Draw a neat and well-labelled diagram for the laboratory preparation of hydrogen.
How would you show that hydrogen is lighter than air?
Starting from zinc how would you obtain hydrogen using Steam.
[Give balanced equation & name the product formed in the case other than hydrogen].
Name a metal which will not react with the reactants above to give hydrogen.
Starting from zinc how would you obtain hydrogen using an alkali.
[Give balanced equation & name the product formed in the case other than hydrogen].
Name a metal which will not react with the reactants above to give hydrogen.
In the laboratory preparation of hydrogen from zinc & dilute hydrocholoric acid – state a reason for the addition of traces of copper [II] sulphate to the reaction medium.
Draw neat labelled diagrams for two different experiments to prove that – hydrogen is lighter than air.
Give balanced equation for the following conversion:
Acidified water to hydrogen – by electrolysis.
Give reason for the following:
In the laboratory preparation of hydrogen from zinc and dilute hydrochloric acid – the zinc used granulated zinc.
Complete and balance the equation:
[Laboratory method]
By action of dilute acid on zinc
Zinc - Zn + 2HCl → _____ + _____ [g]
Give a balanced equation for the following conversions sodium plumbite from lead.
In the laboratory preparation of hydrogen from zinc and dil. acid. Give a reason for the following:
The complete apparatus is air-tight.
In the laboratory preparation of hydrogen from zinc and dil. acid. Give a reason for the following:
The lower end of the thistle funnel should dip below the level of the acid in the flask.
Name the following.
A gaseous reducing agent which is basic in nature.
Select the correct answer from the symbol in the bracket.
The element which forms a diatomic molecule.
The diagram represent the preparation and collection of hydrogen by a standard
laboratory method.
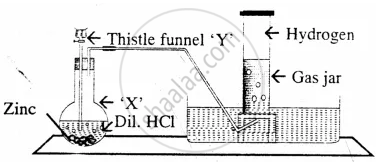
State what is added through the thistle funnel ‘Y’
The diagram represent the preparation and collection of hydrogen by a standard laboratory method.

State, why hydrogen is collected after all the air in the apparatus, is allowed to escape.
