Advertisements
Advertisements
Question
Select the correct answer from the symbol in the bracket.
The element which forms a diatomic molecule.
Options
C
Br
S
P
Solution
Br
APPEARS IN
RELATED QUESTIONS
Indicate which of the following statement is true and which is false:
Nitric acid cannot be used to prepare hydrogen by its action on active metals.
Give reason for the following:
Nitric acid not used for the preparation of hydrogen gas?
Name the following:
A metal which liberates hydrogen only when steam is passed over red hot metal.
How would you show that hydrogen is a non-supporter of combustion?
FILL IN THE BLANK
........................ zinc is preferred over pure zinc in the laboratory preparation of hydrogen.
TRUE \ FALSE
Lead reacts briskly with dilute hydrochloric acid to form hydrogen.
Why is granulated zinc preferred in the laboratory preparation of hydrogen?
Name the impurities present in hydrogen prepared in the laboratory.
How can these impurities be removed?
Give reason:
Apparatus for laboratory preparation of hydrogen should be airtight and away from a naked flame.
How would you show that hydrogen is lighter than air?
In the laboratory preparation of hydrogen from zinc & dilute hydrocholoric acid – state a reason for the addition of traces of copper [II] sulphate to the reaction medium.
Draw neat labelled diagrams for two different experiments to prove that – hydrogen is lighter than air.
Give balanced equation for the following conversion:
Acidified water to hydrogen – by electrolysis.
Give reason for the following:
Copper does not displace hydrogen from dilute hydrochloric acid, but zinc does.
Give a balanced equation for the following conversions sodium aluminate from aluminium.
The diagram represent the preparation and collection of hydrogen by a standard
laboratory method.
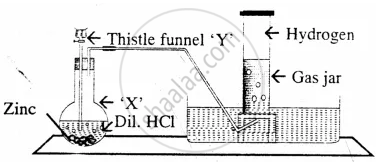
State what is added through the thistle funnel ‘Y’
The diagram represent the preparation and collection of hydrogen by a standard laboratory method.

Name a gas other than hydrogen collected by the same method.
