Advertisements
Advertisements
Question
How can these impurities be removed?
Solution
- The impurities can be removed from hydrogen by passing it through
- Silver nitrate solution to remove arsine and phosphine.
AsH3 + 6AgNO3 → Ag3As + 3AgNO3 + 3HNO3
PH3 + 6AgNO3 → Ag3P + 3AgNO3 + 3HNO3
- Lead nitrate solution to remove hydrogensulphide.
Pb(NO3)2 + H2S → Pbs + 2HNO3
- Caustic potash solution to remove sulphur dioxide, carbon dioxide and oxides of nitrogen.
SO2 + 2KOH → K2SO3 + H2O
CO2 + 2KOH→ K2CO3+ H2O
2NO2 + 2KOH →KNO2 + KNO3 + H2O
- A drying agent used to dry the gas. Common drying agents such as fused calcium chloride, caustic potash stick and phosphorus pentoxide remove water vapor.
So, the gas is purified and dried and then collected over mercury because mercury does not react with it.
APPEARS IN
RELATED QUESTIONS
Indicate which of the following statement is true and which is false:
Nitric acid cannot be used to prepare hydrogen by its action on active metals.
Indicate which of the following statement is true and which is false:
Zinc can liberate hydrogen from water, acid and alkali solution.
Name the following:
A metallic oxide which can be reduced into metal by hydrogen.
Draw a neat and well-labelled diagram for the laboratory preparation of hydrogen.
FILL IN THE BLANK
........................ zinc is preferred over pure zinc in the laboratory preparation of hydrogen.
Give a test to identify hydrogen ?
How would you show that hydrogen is lighter than air?
Starting from zinc how would you obtain hydrogen using an alkali.
[Give balanced equation & name the product formed in the case other than hydrogen].
Name a metal which will not react with the reactants above to give hydrogen.
In the laboratory preparation of hydrogen from zinc & dilute hydrocholoric acid – state a reason for the collecting the hydrogen by downward displacement of water and not air & collecting it after all the air in the apparatus is allowed to escape.
Draw neat labelled diagrams for two different experiments to prove that – hydrogen is lighter than air.
Give balanced equation for the following conversion:
Zinc to sodium zincate – using an alkali.
Give balanced equation for the following conversion:
Acidified water to hydrogen – by electrolysis.
Give reason for the following:
In the laboratory preparation of hydrogen from zinc and dilute hydrochloric acid – the zinc used granulated zinc.
Give a balanced equation for the following conversions sodium plumbite from lead.
Give a balanced equation for the following conversions sodium aluminate from aluminium.
In the laboratory preparation of hydrogen from zinc and dil. acid. Give a reason for the following:
Dilute nitric acid is not preferred as the reactant acid.
The diagram represent the preparation and collection of hydrogen by a standard
laboratory method.
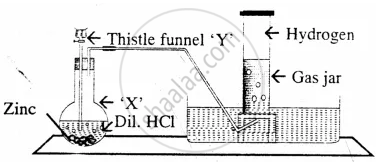
State what is added through the thistle funnel ‘Y’
The diagram represent the preparation and collection of hydrogen by a standard laboratory method.

State what difference will be seen if pure zinc is added in the distillation flask ‘X’ instead of granulated zinc.
The diagram represent the preparation and collection of hydrogen by a standard laboratory method.

Name a gas other than hydrogen collected by the same method.
