Advertisements
Advertisements
Question
How can the following conversions be brought about:
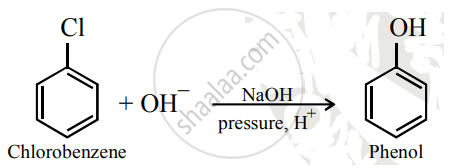
Chlorobenzene to phenol
Solution
Chlorobenzene to phenol
APPEARS IN
RELATED QUESTIONS
A solution of sodium chloride has no effect on litmus, but a solution of zinc chloride turns blue litmus red.
How can the following conversions be brought about:
(i) Acetic acid to methyl cyanide.
(ii) Acetaldehyde to formaldehyde.
(iii) Nitrobenzene to 2, 4, 6 tribromoaniline.
Identify the reagents A, B, C, D, E and F required for the following conversion:
\[\ce{C6H5NO2->[A]C6H5NH2->[B]C6H5N2+Cl- ->[C]C6H5Cl->[D]C6H5OH->[H2SO4]E + F}\]
Complete the following reaction:
\[\begin{array}{cc}
\ce{CH3}\phantom{..............}\\
|\phantom{..............}\phantom{...}\\
\ce{CH3-CH-COOH->[(i) Br2/Red P4][(ii) H2O]}\\
\phantom{.......}\
\end{array}\]
The acid which doesn't contain COOH group is
Formic acid when heat with H2SO4 gives
The oxidation of toluene with hot KMnO4 gives ______.
Why formic acid is stronger than acetic acid?
Carboxylic acid present in curd is ______.
