Advertisements
Advertisements
Question
How is 1-propoxypropane synthesised from propan-1-ol? Write mechanism of this reaction.
Solution
\[\ce{\underset{Propanol}{CH3CH2CH2OH} ->[?]\underset{Propoxypropane}{(CH3CH2CH2)2O}}\]
This reaction can be brought about as –
\[\ce{\underset{Propanol}{CH3CH2CH2OH} + SOCl2 -> CH3CH2CH2Cl}\]
\[\ce{2CH3CH2CH2CH2OH + \underset{(Metal)}{2Na} -> 2CH3CH2CH2CH2\overset{—}{O}N\overset{+}{a} + H2}\]
\[\ce{\underset{1-Chloropropane}{CH3CH2CH2Cl} + \underset{Sodium propoxide}{CH3CH2CH2\overset{—}{O}}N\overset{+}{a} -> \underset{Propoxypropane}{(CH3CH2CH2)2O}}\]
Mechanism:
SN2 attack of propoxide on the halide.
APPEARS IN
RELATED QUESTIONS
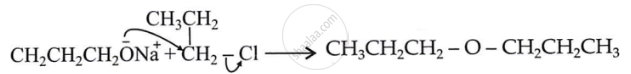
Explain the mechanism of the following reaction:
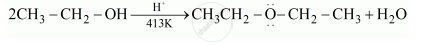
Write the name of the reagent and the equation for the preparation of the following ether by Williamson’s synthesis:
2-Methoxy-2-methylpropane
Illustrate with examples the limitations of Williamson synthesis for the preparation of certain types of ethers.
Write the equations for the following reaction:
Tert butyl chloride is treated with sodium ethoxide.
The major product [B] in the following reactions is:
\[\begin{array}{cc}\ce{CH3}\phantom{..................................}\\|\phantom{.....................................}\\\ce{CH3 - CH2 - CH - CH2 - OCH2 - CH3 ->[HI][Heat] [A] alcohol ->[H2SO4][\Delta] [B]}\end{array}\]
HBr reacts with \[\ce{CH2 = CH - OCH3}\] under anhydrous conditions at room temperature to give ______.
Write the mechanism of the following reaction:
\[\ce{2CH3CH2OH ->[H^+][413 K] CH3-CH2-O-CH2-CH3 + H2O}\]
Write the name of reagent and equation for the preparation of the following ether by Williamson’s synthesis:
2-Methoxy-2-methylpropane
Write the name of reagent and equation for the preparation of the following ethers by Williamson’s synthesis:
2-Methoxy-2-methylpropane
Write the name of the reagent and equation for the preparation of the following ether by Williamson’s synthesis:
2-Methoxy-2-methylpropane
Predict the major product, when 2-methyl but -2-ene is converted into an alcohol in the following method.
Acid catalysed hydration
What will be the product (X and A)for the following reaction
\[\ce{acetylchloride->[i)CH3MgBr][ii)H3O+]X ->[acidk2crp3]A}\]
Write the names of reagents and equations for the preparation of the following ether by Williamson’s synthesis:
2-Methoxy-2-methylpropane
Short Answer Question.
Identify the product (s) is/are formed when 1 – methoxy propane is heated with excess HI. Name the mechanism involved in the reaction.
Give the structure and IUPAC name of metamers of 2-methoxy propane
Write the name of reagent and equation for the preparation of the following ether by Williamson’s synthesis:
2-Methoxy-2-methylpropane
Write the name of reagent and equation for the preparation of the following ether by Williamson’s synthesis:
2-Methoxy-2-methylpropane
