Advertisements
Advertisements
Question
How will you convert benzoic acid to m-bromobenzoic acid?
Solution
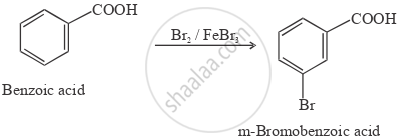
APPEARS IN
RELATED QUESTIONS
Draw the structure of the semicarbazone of ethanal.
Write the products formed when CH3CHO reacts with the following reagents : HCN
Arrange the following compound in increasing order of its reactivity in nucleophilic addition reactions.
Ethanal, Propanal, Propanone, Butanone.
Hint: Consider steric effect and electronic effect.
Predict the products formed when cyclohexanecarbaldehyde reacts with the following reagents.
PhMgBr and then H3O+
Predict the products formed when cyclohexanecarbaldehyde reacts with the following reagents.
Excess ethanol and acid
How are the following compounds prepared?
acetophenone from benzene
How are the following compounds prepared?
benzaldehyde from benzoyl chloride
Write balanced chemical equations for action of ammonia on - acetone
Carboxylic acids contain carbonyl group but do not show the nucleophilic addition reaction like aldehydes or ketones. Why?
A Idol condensation will not be observed in
The most stable reagent for the conversion of R – CH2OH → RCHO is
Which among the following is most reactive to give nucleophilic addition?
Write the name of product formed, when acetone is treated with 2, 4-dinitrophenyl hydrazine.
What is the action of sodium hypoiodite on acetone?
Arrange the following in the increasing order of their property indicated:
Ethanal, Propanone, Propanal, Butanone (reactivity towards nucleophilic addition)
What happens when propanone is treated with CH3MgBr and then hydrolysed?
The increasing order of the following compounds towards HCN addition is:
| (i) | 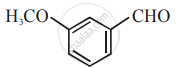 |
| (ii) | 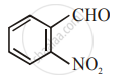 |
| (iii) | 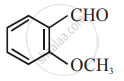 |
| (iv) | 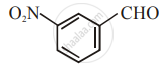 |
In the following reaction
\[\ce{Carbonyl compound + MeOH <=>[HCl] acetal}\]
Rate of the reaction is the highest for ______.
The product of following reaction is
\[\ce{CH3 - CH = CH - CH2 - CHO ->[i) LiAlH4][ii) H3O+]}\] ______?
Which of the following is most reactive in nucleophilic addition reactions?
Aldehydes and ketones react with hydroxylamine to form ______.
Why dissociation of HCN is suppressed by the addition of HCL?
Draw structures of the following derivative.
The ethylene ketal of hexan-3-one
The product of the following reaction is
\[\begin{array}{cc}
\ce{O}\phantom{.........}\\
||\phantom{.........}\\
\ce{C2H5 - C - CH3 ->[H2/Ni][\Delta] \phantom{..}?}\end{array}\]
Draw the structure of the following derivative.
The ethylene ketal of hexan-3-one
Draw structure of the following derivative.
The ethylene ketal of hexan-3-one
Give an example of the reaction in the following case.
Oxime
Give an example of the reaction in the following case.
Imine
