Advertisements
Advertisements
Question
Identify the term/substance in the following:
The tendency of an atom to attract electrons to itself when combined in a compound.
Solution
The tendency of an atom to attract electrons to itself when combined in a compound: Electronegativity
APPEARS IN
RELATED QUESTIONS
Name the elements with highest and lowest electronegativity ?
Arrange the elements of group 17 and group 1 according to the given conditions.
Decreasing electro negativity.
The tendency of an atom to attract electrons itself when combined in a covalent compound.
Study the extract of the Periodic Table given below and answer the questions that follow. Give the alphabet corresponding to the element in question. DO NOT repeat an element. 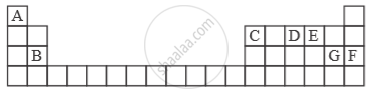
Which element forms electrovalent compound with G?
Among the elements of the second period, Li to Ne, pick out the element with the highest electro negativity
Fill in the blank
Most electronegative elements belong to ______ group.
Fill in the blank
Reactivity ______ down the gruop for alkali metals with increase in electropositive character
Explain
Electronegativity of Cl is higher than S.
The electronegativities (according to Pauling) of the elements in period 3 of the periodic table are as follows with elements arranged in alphabetical order:
| Al | Cl | Mg | Na | P | S | Si |
| 1.5 | 3.0 | 1.2 | 0.9 | 2.1 | 2.5 | 1.8 |
Arrange the elements in the order in which they occur in the periodic table from left to right.
(The group 1 element first, followed by the group 2 element and so on, up to group 7).
Arrange the following as per instruction given in the bracket.
Cs, Na, Li, K, Rb (decreasing electronegativity)
