Advertisements
Advertisements
Question
If the radius of the octachedral void is r and radius of the atoms in close packing is R, derive relation between r and R.
Solution
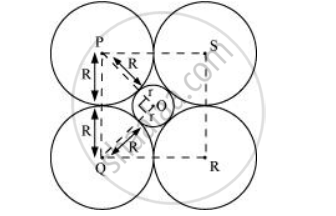
A sphere with centre O, is fitted into the octahedral void as shown in the above figure. It can be observed from the figure that ΔPOQ is right-angled
∠POQ = 900
Now, applying Pythagoras theorem, we can write:
`PQ^2 = PO^2 + OQ^2`
`=>(2R)^2 = (R+r)^2 + (R+r)^2`
`=>(2R)^2 = 2(R+r)^2`
`=rR^2 = (R+r)^2`
`=>sqrt2R = R +r`
`=>r =sqrt2R -R`
`=> r = (sqrt2-1)R`
`=>r = 0.414R`
APPEARS IN
RELATED QUESTIONS
What is the formula of a compound in which the element Y forms hcp lattice and atoms of X occupy 2/3rd of tetrahedral voids?
How many tetrahedral voids can exist per unit cell in a hexagonal close packing sphere?
The unit cell of a substance has cations A+ at the corners of the unit cell and the anions B− at the center. The simplest formula of the substance is ____________.
What is the coordination number of sodium in Na2O?
If AgI crystallises in zinc blende structure with I– ions at lattice points. What fraction of tetrahedral voids is occupied by Ag+ ions?
Which set of following characteristics for ZnS crystal is correct?
In which of the following crystals alternate tetrahedral voids are occupied?
The number of octahedral voids present in a lattice is A. The number of closed packed articles, the number of tetrahedral voids generated is B the number of closed packed particles:
If Germanium crystallises in the same way as diamond, then which of the following statement is not correct?
The coordination number of Y will be in the XY types of crystal:
The number of tetrahedral voids per unit cell in NaCl crystal is:
(i) 4
(ii) 8
(iii) twice the number of octahedral voids.
(iv) four times the number of octahedral voids.
Show that in a cubic close packed structure, eight tetrahedral voids are present per unit cell.
A solid compound XY has Nacl structure. If the radium of cation (X+) is 100 pm, the radium of anion (r–) will be:-
In which of the following structures coordination number for cations and anions in the packed structure will be same?
