Advertisements
Advertisements
Question
Show that in a cubic close packed structure, eight tetrahedral voids are present per unit cell.
Solution
In ccp structure unit cell is divided into 8 small cubes. Each small cube has atoms at alternative corners [Figure]. In all, each small cube has 4 atoms. When joined to one another, they make a regular tetrahedron. Thus, there is one tetrahedral void in each small cube and 8 tetrahedral void in total. Each of the eight small cubes has one void in one unit cell of ccp structure. We know that ccp structure has four atoms per unit cell. Thus the number of tetrahedral voids is twice the number of atoms.
(a)
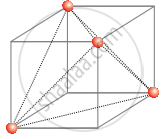
(b)
Again, the number of tetrahedral voids in ccp structure is equal to twice the total number of atoms.
APPEARS IN
RELATED QUESTIONS
What is the formula of a compound in which the element Y forms hcp lattice and atoms of X occupy 2/3rd of tetrahedral voids?
How will you distinguish between the following pairs of terms: Tetrahedral and octahedral voids
If the radius of the octachedral void is r and radius of the atoms in close packing is R, derive relation between r and R.
How many tetrahedral voids can exist per unit cell in a hexagonal close packing sphere?
Total number of voids in 0.5 mole of a compound which forms hexagonal close packed structure is ____________.
What is the coordination number of sodium in Na2O?
If AgI crystallises in zinc blende structure with I– ions at lattice points. What fraction of tetrahedral voids is occupied by Ag+ ions?
In the hexagonal close-packed structure of a metallic lattice, the number of nearest neighbours of a metallic atom is ____________.
The coordination number of Y will be in the XY types of crystal:
Which of the following is not true about the voids formed in 3 dimensional hexagonal close packed structure?
(i) A tetrahedral void is formed when a sphere of the second layer is present above triangular void in the first layer.
(ii) All the triangular voids are not covered by the spheres of the second layer.
(iii) Tetrahedral voids are formed when the triangular voids in the second layer lie above the triangular voids in the first layer and the triangular shapes of these voids do not overlap.
(iv) Octahedral voids are formed when the triangular voids in the second layer exactly overlap with similar voids in the first layer.
