Advertisements
Advertisements
Question
Which of the following is not true about the voids formed in 3 dimensional hexagonal close packed structure?
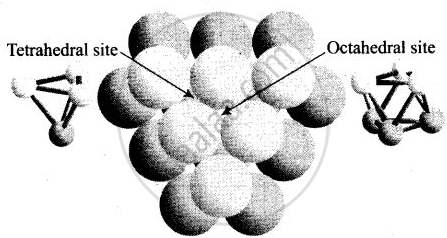
(i) A tetrahedral void is formed when a sphere of the second layer is present above triangular void in the first layer.
(ii) All the triangular voids are not covered by the spheres of the second layer.
(iii) Tetrahedral voids are formed when the triangular voids in the second layer lie above the triangular voids in the first layer and the triangular shapes of these voids do not overlap.
(iv) Octahedral voids are formed when the triangular voids in the second layer exactly overlap with similar voids in the first layer.
Solution
(iii) Tetrahedral voids are formed when the triangular voids in the second layer lie above the triangular voids in the first layer and the triangular shapes of these voids do not overlap.
(iv) Octahedral voids are formed when the triangular voids in the second layer exactly overlap with similar voids in the first layer.
Explanation:
Tetrahedral voids are formed when the triangular void in the second layer lie exactly above the triangular voids in the first layer and the triangular shape of these voids oppositely overlap.
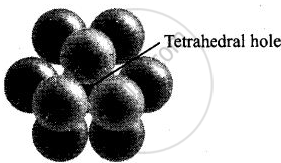
Octahedral voids are formed when triangular void of second layer is not exactly overlap with similar void in first layer.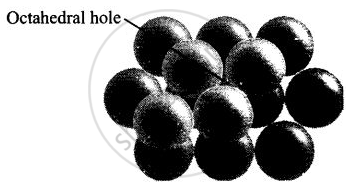
APPEARS IN
RELATED QUESTIONS
What is the formula of a compound in which the element Y forms hcp lattice and atoms of X occupy 2/3rd of tetrahedral voids?
How many tetrahedral voids can exist per unit cell in a hexagonal close packing sphere?
The unit cell of a substance has cations A+ at the corners of the unit cell and the anions B− at the center. The simplest formula of the substance is ____________.
If AgI crystallises in zinc blende structure with I– ions at lattice points. What fraction of tetrahedral voids is occupied by Ag+ ions?
In which of the following crystals alternate tetrahedral voids are occupied?
If Germanium crystallises in the same way as diamond, then which of the following statement is not correct?
In the cubic close packing, the unit cell has ______.
In which of the following arrangements octahedral voids are formed?
(i) hcp
(ii) bcc
(iii) simple cubic
(iv) fcc
Show that in a cubic close packed structure, eight tetrahedral voids are present per unit cell.
A solid compound XY has Nacl structure. If the radium of cation (X+) is 100 pm, the radium of anion (r–) will be:-
