Advertisements
Advertisements
प्रश्न
If the radius of the octachedral void is r and radius of the atoms in close packing is R, derive relation between r and R.
उत्तर
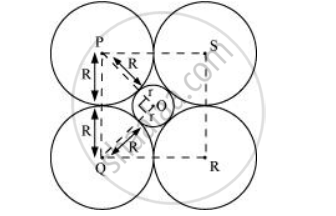
A sphere with centre O, is fitted into the octahedral void as shown in the above figure. It can be observed from the figure that ΔPOQ is right-angled
∠POQ = 900
Now, applying Pythagoras theorem, we can write:
`PQ^2 = PO^2 + OQ^2`
`=>(2R)^2 = (R+r)^2 + (R+r)^2`
`=>(2R)^2 = 2(R+r)^2`
`=rR^2 = (R+r)^2`
`=>sqrt2R = R +r`
`=>r =sqrt2R -R`
`=> r = (sqrt2-1)R`
`=>r = 0.414R`
APPEARS IN
संबंधित प्रश्न
How will you distinguish between the following pairs of terms: Tetrahedral and octahedral voids
The unit cell of a substance has cations A+ at the corners of the unit cell and the anions B− at the center. The simplest formula of the substance is ____________.
Total number of voids in 0.5 mole of a compound which forms hexagonal close packed structure is ____________.
A compound is formed by two elements A and B. The element B forms cubic close packing and atoms of A occupy `1/3"rd"` of tetrahedral voids. The formula of this compound is ____________.
What is the coordination number of sodium in Na2O?
The Ca2+ and F– are located in CaF2 crystal, respectively at face centered cubic lattice points and in ____________.
If AgI crystallises in zinc blende structure with I– ions at lattice points. What fraction of tetrahedral voids is occupied by Ag+ ions?
Which set of following characteristics for ZnS crystal is correct?
The number of octahedral voids present in a lattice is A. The number of closed packed articles, the number of tetrahedral voids generated is B the number of closed packed particles:
In the hexagonal close-packed structure of a metallic lattice, the number of nearest neighbours of a metallic atom is ____________.
In which of the following arrangements octahedral voids are formed?
(i) hcp
(ii) bcc
(iii) simple cubic
(iv) fcc
The coordination number of Y will be in the XY types of crystal:
Which of the following is not true about the voids formed in 3 dimensional hexagonal close packed structure?
(i) A tetrahedral void is formed when a sphere of the second layer is present above triangular void in the first layer.
(ii) All the triangular voids are not covered by the spheres of the second layer.
(iii) Tetrahedral voids are formed when the triangular voids in the second layer lie above the triangular voids in the first layer and the triangular shapes of these voids do not overlap.
(iv) Octahedral voids are formed when the triangular voids in the second layer exactly overlap with similar voids in the first layer.
Show that in a cubic close packed structure, eight tetrahedral voids are present per unit cell.
A solid compound XY has Nacl structure. If the radium of cation (X+) is 100 pm, the radium of anion (r–) will be:-
