Advertisements
Advertisements
Question
In the experimental set-up to show that "the germinating seeds give out carbon dioxide", answer the following questions:
(i) Why do we keep the conical flask airtight?
(ii) Name the substance kept in the small test tube inside the conical flask. Write its role.
(iii) Why does water rise in the delivery tube?
Solution
(iii) The rise in the water level in the tube indicates the production of CO2 by the germinating seeds. The KOH absorbs the CO2 produced by the germinating seeds and thus creates a partial vacuum in the flask. The air present in the glass bent tube moves into the conical flask, as a result of which the water is pulled further up in the bent tube.
APPEARS IN
RELATED QUESTIONS
Lime water turns milky when __________ gas is passed through it.
(a) H2
(b) CO
(c) CO2
(d) SO2
What colour do the following indicators turn when added to a base or alkali (such as sodium hydroxide)?
red cabbage extract
What happens when dilute hydrochloric acid is added to sodium carbonate? Write a balanced chemical equation of the reaction involved.
What is meant by strong bases and weak bases? Classify the following into strong bases and weak bases:
NH4OH, Ca(OH)2, NaOH, KOH, Mg(OH)2
Write the main difference between an acid and a base.
Choose the correct option from given alternative:
When a small amount of acid is added to water, the phenomena which occur are:
(A) Dilution
(B) Neutralization
(C) Formation of H3O+ ions
(D) Salt formation
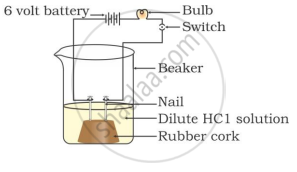
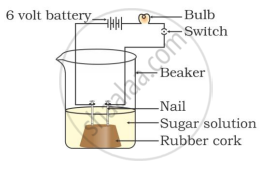
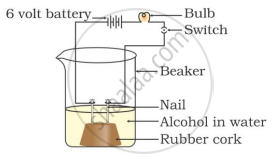
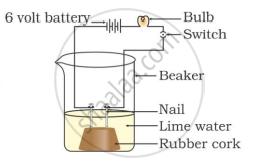
In which of the following setups would the bulb glow?
Which of the following is(are) true when HCl (g) is passed through water?
- It does not ionise in the solution as it is a covalent compound.
- It ionises in the solution
- It gives both hydrogen and hydroxyl ions in the solution
- It forms hydronium ion in the solution due to the combination of hydrogen ion with water molecule
A metal carbonate X on reacting with an acid gives a gas which when passed through a solution Y gives the carbonate back. On the other hand, a gas G that is obtained at anode during electrolysis of brine is passed on dry Y, It gives a compound Z, used for disinfecting drinking water. Identity X, Y, G and Z.
- A compound 'A' with a molecular formula of \[\ce{C2H4O2}\] reacts with a base to give salt and water. Identify 'A', state its nature and the name of the functional group it possesses. Write chemical equation for the reaction involved.
- When the above stated compound 'A' reacts with another compound 'B' having molecular formula \[\ce{C2H6O}\] in the presence of an acid, a sweet smelling compound is 'C' formed.
- Identify 'B' and 'C'.
- State the role of acid in this reaction.
- Write chemical equation for the reaction involved.