Advertisements
Advertisements
Question
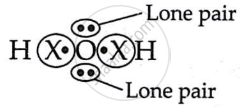
In the molecule of water, oxygen atom has ______.
Options
One shared pair of electrons
Three shared pairs of electrons
Two lone pairs of electrons
One lone pair of electrons
Solution
In the molecule of water, oxygen atom has two lone pairs of electrons.
Explanation:
A water molecule has 2H atoms and 10 atoms. O has an electrical configuration of 2, 6 and requires 2 electrons to complete its octet.
∴ it shares 2e− with 2H−atoms
APPEARS IN
RELATED QUESTIONS
What do you understand by lone pair of electrons ?
Draw an electron dot diagram for the formation of the following. State the type of bonding present in them.
Hydronium ion.
Give an example for each of the following statement.
A compound in which coordinate bond is formed.
Draw the electron dot structure for the following:
Ammonium ion
[At. No. : N = 7, H = 1]
