(English Medium)
Academic Year: 2023-2024
Date & Time: 11th March 2024, 11:00 am
Duration: 2h
Advertisements
- Answers to this Paper must be written on the paper provided separately.
- You will not be allowed to write during the first 15 minutes.
- This time is to be spent in reading the question paper.
- The time given at the head of this Paper is the time allowed for writing the answers.
- Section A is compulsory. Attempt any four questions from Section B.
- The intended marks for questions or parts of questions are given in brackects [ ].
The unsaturated hydrocarbons undergo ______.
a substitution reaction
an oxidation reaction
an addition reaction
none of the above
redox reaction
Chapter: [0.09] Organic Chemistry
In the 2nd period Neon has maximum Ionization Potential because ______.
It has unstable electronic configuration.
It easily accepts electrons.
It easily loses electrons.
The outer most shell is completely filled.
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
Copper, zinc, and Tin are the metals alloyed to form ______.
Duralumin
Brass
Bronze
Solder
Chapter: [0.07] Metallurgy
The metal hydroxide which reacts with both acids and alkalis to form salt and water is ______.
Calcium hydroxide
Magnesium hydroxide
Aluminium hydroxide
Ferric hydroxide
Chapter: [0.04] Analytical Chemistry
Reaction of an alcohol with a carboxylic acid in the presence of concentrated \[\ce{H2SO4}\] is termed as ______.
Halogenation
Esterification
Hydrogenation
Dehydrohalogenation
Chapter: [0.09] Organic Chemistry
Conversion of ethanol to ethene by the action of concentrated sulphuric acid is an example of _________.
Dehydration
Dehydrogenation
Dehydrohalogenation
Hydrolysis
Chapter: [0.09] Organic Chemistry
The oxidizing agent in the equation \[\ce{S + 2H2SO4 -> 3SO2 + 2H2O}\] is ______.
Sulphur
Sulphuric acid
Sulphur dioxide
Water
Chapter: [0.06] Electrolysis
Electron Affinity is maximum in ______.
Mg
Ar
Li
Br
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
The compound that is not a constituent of the electrolytic mixture used in Hall-Heroult's process is ______.
\[\ce{Al2O3}\]
\[\ce{NaAlO2}\]
\[\ce{Na3AlF6}\]
\[\ce{CaF2}\]
Chapter: [0.07] Metallurgy
On passing ammonia gas over heated copper oxide for some time, a reddish-brown residue is left behind. What property of ammonia is demonstrated here?
Basic property
Oxidising property
Reducing property
Acidic property
Chapter: [0.08199999999999999] Ammonia
Rotten egg smell is due to the liberation of ______.
\[\ce{HCI}\] gas
\[\ce{H2S}\] gas
\[\ce{Cl2}\] gas
\[\ce{SO2}\] gas
Chapter: [0.1] Practical Work
Ammonia gas is collected by downward displacement of air since ammonia is ______.
Very slightly soluble in water
Heavier than air
Lighter than air
Insoluble in water
Chapter: [0.08199999999999999] Ammonia [0.1] Practical Work
Which of the following would occupy 22.4 litres at S.T.P.?
- 32 g of oxygen gas
- 2 moles of hydrogen gas
- 6.022 × 1023 molecules of ammonia
[Atomic weights: O = 16, H = 1, N = 14]
1 and 2
1 and 3
2 and 3
1, 2 and 3
Chapter: [0.05] Mole Concept and Stoichiometry
In the molecule of water, oxygen atom has ______.
One shared pair of electrons
Three shared pairs of electrons
Two lone pairs of electrons
One lone pair of electrons
Chapter: [0.02] Chemical Bonding
A mineral from which the metal can be extracted economically and conveniently is known as ______.
Matrix
Ore
Flux
Alloy
Chapter: [0.07] Metallurgy
The following sketch represents the electroplating of an Iron cup with Nickel metal.
Study the diagram and answer the following questions:
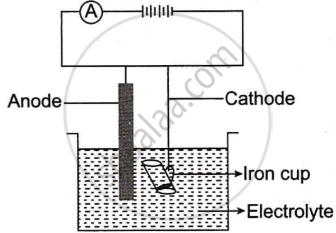
- During electroplating, the iron cup is placed at the cathode. Why?
- Name the ion that must be present in the electrolyte.
- State one condition that is necessary to ensure that the deposit is smooth, firm and even.
- Write the reaction taking place at the cathode.
- What change would you observe at the anode?
Chapter: [0.07] Metallurgy
Match the Column A with Column B.
| Column A | Column B | ||
| (a) | Water | 1. | Lithium |
| (b) | Alkali metal | 2. | Iodine |
| (c) | Halogen | 3. | Covalent compound |
| (d) | Calcium oxide | 4. | Acetic acid |
| (e) | Weak acid | 5. | Ionic compound |
| 6. | Sulphuric acid | ||
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
The salt prepared by the method of direct combination is _______.
Iron (II) chloride \[\ce{(FeCl2)}\]
Iron (III) chloride \[\ce{(FeCl3)}\]
Chapter: [0.03] Study of Acids, Bases and Salts
The metallic oxide which can be reduced by using common reducing agents is ______.
\[\ce{Fe2O3}\]
\[\ce{Al2O3}\]
Chapter: [0.06] Electrolysis
The metal nitrate which on thermal decomposition forms a black residue is ______.
zinc nitrate
copper nitrate
Chapter: [0.1] Practical Work
During the electrolysis of copper sulphate solution, if ______ is used as electrodes, the colour of the electrolyte does not fade.
copper
platinum
Chapter: [0.06] Electrolysis
The process of heating the concentrated ore in a limited supply or absence of air is ______.
Roasting
Calcination
Chapter: [0.07] Metallurgy
State the term for the following:
The group obtained by removing one hydrogen atom from the parent alkane.
Chapter: [0.09] Organic Chemistry
State the term for the following:
Two metal plates or wires through which the current enters and leaves the electrolytic cell.
Chapter: [0.06] Electrolysis
State the term for the following:
The amount of substance which contains the same number of units as the number of atoms in carbon-12.
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
State the term for the following:
The tendency of an atom to pull a shared pair of electrons towards itself in a compound.
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
State the term for the following:
The formula which represents the simplest ratio between the atoms of elements present in a compound.
Chapter: [0.05] Mole Concept and Stoichiometry
Give the IUPAC name of the organic compound represented by the structural formula given below:
\[\begin{array}{cc}
\ce{H}\phantom{...}\ce{Cl}\phantom{..}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\\
\ce{H - C - C - C - C - C - H}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\\
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{Cl}\phantom{..}\ce{H}\phantom{...}\ce{H}\end{array}\]
Chapter: [0.09] Organic Chemistry
Give the IUPAC name of the organic compound represented by the structural formula given below:
\[\begin{array}{cc}
\phantom{.}\ce{H\phantom{...}H\phantom{...}O}\phantom{..}\\
\phantom{.}|\phantom{....}|\phantom{....}||\phantom{..}\\
\ce{H - C - C - C - OH}\\
\phantom{}|\phantom{....}|\phantom{......}\\
\phantom{}\ce{H\phantom{...}H}\phantom{......}
\end{array}\]
Chapter: [0.09] Organic Chemistry
Draw the structural diagram for the following organic compound:
3-methyl pentane
Chapter:
Advertisements
Give the structural formula of the following organic compound:
Methanal
Chapter: [0.09] Organic Chemistry
Rewrite the following statement by adding the correct word, as shown in the example:
|
Example: Given Statement: Ammonia changes moist red litmus to blue. Correct Statement: Aqueous ammonia changes moist red litmus to blue. |
Sulphuric acid acts as a dehydrating agent.
Chapter: [0.084] Sulphuric Acid
Rewrite the following statement by adding the correct word, as shown in the example:
|
Example: Given Statement: Ammonia changes moist red litmus to blue. Correct Statement: Aqueous ammonia changes moist red litmus to blue. |
Ammonia reacts with chlorine to give ammonium chloride and nitrogen.
Chapter: [0.08199999999999999] Ammonia [0.1] Practical Work
Identify only the anion present in the following compound:
The compound, on heating, produces a colourless, odourless gas which turns lime water milky and has no effect on acidified potassium dichromate solution.
Chapter: [0.1] Practical Work
Identify only the anion present in the following compound:
The solution of the compound which on treating with concentrated sulphuric acid and freshly prepared ferrous sulphate solution produces a brown ring.
Chapter: [0.1] Practical Work
Mohan has three solutions P, Q and R having a pH of 13, 5 and 2 respectively. Which of the above solutions P, Q or R will react with magnesium to liberate hydrogen gas?
Chapter: [0.03] Study of Acids, Bases and Salts [0.1] Practical Work
Mohan has three solutions P, Q and R having a pH of 13, 5 and 2 respectively. Which of the above solutions P, Q or R will liberate ammonia gas when it reacts with ammonium chloride?
Chapter: [0.03] Study of Acids, Bases and Salts [0.1] Practical Work
Mohan has three solutions P, Q and R having a pH of 13, 5 and 2 respectively. Which of the above solutions P, Q or R will contain molecules as well as ions?
Chapter: [0.03] Study of Acids, Bases and Salts [0.1] Practical Work
The following table is related to an industrial process of an acid.
| Name of the process | Reactant | Catalyst | Final product |
| (a) | SO2 + O2 | (b) | (c) |
Identify (a), (b) and (c).
Chapter: [0.081] Hydrogen Chloride
Define the term.
Molar volume
Chapter: [0.05] Mole Concept and Stoichiometry
Define normal salt.
Chapter: [0.03] Study of Acids, Bases and Salts
Draw the electron dot structure of Methane molecule.
[Atomic number: N = 7, C = 6, H = 1]
Chapter: [0.02] Chemical Bonding
Draw the electron dot structure of Nitrogen molecule.
[Atomic number: N = 7, C = 6, H = 1]
Chapter: [0.02] Chemical Bonding
Complete and balance the following equation:
\[\ce{Al2O3 + NaOH ->}\]
Chapter: [0.05] Mole Concept and Stoichiometry
Complete and balance the following equation:
\[\ce{C2H5COONa + NaOH ->[\Delta][CaO]}\]
Chapter: [0.05] Mole Concept and Stoichiometry
Complete and balance the following equation:
\[\ce{C2H4Br2 +alcoholic KOH ->[\Delta]}\]
Chapter: [0.05] Mole Concept and Stoichiometry
The compound which does not have a double bond in its structure.
Ethene
Ethanoic acid
Ethanol
Methanal
Chapter: [0.09] Organic Chemistry
The compound in its pure form turns into an ice like solid on cooling.
Ethene
Ethanoic acid
Ethanol
Methanal
Chapter: [0.09] Organic Chemistry
The compound which is used for artificial ripening of fruits.
Ethene
Ethanoic acid
Ethanol
Methanal
Chapter: [0.09] Organic Chemistry
Name the main constituent metal in the following alloy:
Duralumin
Chapter: [0.07] Metallurgy
Name the main constituent metal in the following alloy:
Stainless steel
Chapter: [0.07] Metallurgy
Differentiate between the following pairs based on the odourless gas which turns lime water milky and the criteria given:
Sulphuric acid and Nitric acid (using barium chloride solution)
Chapter: [0.083] Nitric Acid
Differentiate between the following pairs based on the criteria given:
Unsaturated and Saturated hydrocarbons (type of bond present)
Chapter: [0.09] Organic Chemistry
Calcium carbonate react with dilute hydrochloric acid as given below:
\[\ce{CaCO3 + 2HCl -> CaCl2 + H2O + CO2}\]
What is the mass of 5 moles of calcium carbonate? (Relative molecular mass of calcium carbonate is 100)
Chapter: [0.05] Mole Concept and Stoichiometry
Calcium carbonate react with dilute hydrochloric acid as given below:
\[\ce{CaCO3 + 2HCl -> CaCl2 + H2O + CO2}\]
How many moles of \[\ce{HCl}\] will react with 5 moles of calcium carbonate?
Chapter: [0.05] Mole Concept and Stoichiometry
Advertisements
Calcium carbonate react with dilute hydrochloric acid as given below:
\[\ce{CaCO3 + 2HCl -> CaCl2 + H2O + CO2}\]
What is the volume of carbon dioxide liberated at S.T.P. at the same time?
Chapter: [0.05] Mole Concept and Stoichiometry
Identify the gas evolved in the following reaction:
Methane undergoes complete combustion.
Chapter: [0.09] Organic Chemistry
Identify the gas evolved in the following reaction:
Copper carbonate is heated.
Chapter: [0.1] Practical Work
Identify the gas evolved in the following reaction:
\[\ce{MnO2}\] reacts with concentrated \[\ce{HCl}\].
Chapter: [0.081] Hydrogen Chloride [0.1] Practical Work
X - \[\ce{HCl <=> H^{1+} + Cl-}\] (in solution state)
Y - \[\ce{PbBr2 <=> Pb^{2+} + 2Br^{1-}}\] (in molten state)
From the above reaction X or Y, identify the reaction which exhibit:
electrolytic dissociation
Chapter: [0.06] Electrolysis
X - \[\ce{HCl <=> H^{1+} + Cl-}\] (in solution state)
Y - \[\ce{PbBr2 <=> Pb^{2+} + 2Br^{1-}}\] (in molten state)
From the above reaction X or Y, identify the reaction which exhibit:
ionization
Chapter: [0.06] Electrolysis
Give a reason for Inert gases do not form ions.
Chapter: [0.02] Chemical Bonding
Give reason for the following:
Covalent compounds have a low melting and boiling point.
Chapter: [0.02] Chemical Bonding
Arrange the following as per the instruction given in the bracket:
Carbon, Fluorine, Beryllium (decreasing order of atomic size).
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
Arrange the following as per the instruction given in the bracket:
Sulphuric acid, Phosphoric acid, Acetic acid (increasing order of number of replaceable H atoms per molecule).
Chapter: [0.084] Sulphuric Acid
Arrange the following as per the instruction given in the bracket:
Potassium, Lithium, Sodium (increasing order of ionization potential).
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
Identify the following:
An element in Period 1 which can be placed in both Group 1 and Group 17 of the Periodic Table.
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
Identify the following:
The element having electronic configuration 2, 8, 6.
Chapter: [0.084] Sulphuric Acid
Identify the following:
The most electronegative element of Period 3.
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
Rita was given an unknown salt for identification. She prepared a solution of the salt and divided it into two parts.
- To the first part of the salt solution, she added a few drops of ammonium hydroxide and obtained a reddish-brown precipitate.
- To the second part of the salt solution, she added a few drops of silver nitrate solution and obtained a white precipitate.
Name:
- The cation present and
- The anion present in the salt given for identification.
Chapter: [0.04] Analytical Chemistry
Carbon tetrachloride is a ______ covalent molecule.
Polar
Non-polar
Chapter: [0.02] Chemical Bonding
During electrolysis of acidulated water, the gas liberated at the anode is ______.
Oxygen
Hydrogen
Chapter: [0.06] Electrolysis
Ammonia burns in oxygen, as shown below.
\[\ce{4NH3 + 3O2 -> 2N2 + 6H2O}\]
If 240 cc of ammonia is burnt in 300 cc of oxygen, find out the composition of the resultant gaseous mixture at room temperature.
Chapter: [0.08199999999999999] Ammonia
The following table shows the electronic configuration of the atoms A, B, C and D.
| Element | A | B | C | D |
| Electronic configuration | 2, 8, 8, 2 | 2, 6 | 2, 8, 7 | 2, 4 |
- Write the formula of the compound formed between:
- A and B
- D and C
- Which of the above elements will exhibit catenation?
Chapter: [0.02] Chemical Bonding
The ore which can be concentrated by magnetic separation.
Zinc blende
C2H2
Calamine
CH
Haematite
Chapter: [0.07] Metallurgy
Empirical formula of Ethyne.
Zinc blende
C2H2
Calamine
CH
Haematite
Chapter: [0.05] Mole Concept and Stoichiometry
Give a balanced equation for the following reaction:
Copper reacts with concentrated nitric acid.
Chapter: [0.083] Nitric Acid
Write the equation for the reaction:
Aluminum, Nitride and Water.
Chapter: [0.083] Nitric Acid
Match the salts underlined in Column A with the most suitable method of preparation given in Column B.
| Column A | Column B | ||
| (a) | \[\ce{ZnCl2 \text{from} Zn}\] | 1. | Precipitation |
| (b) | \[\ce{KNO3 \text{from} KOH}\]. | 2. | Direct combination |
| (c) | \[\ce{CaCO3 \text{from} CaCl2}\]. | 3. | Displacement reaction |
| 4. | Neutralization |
Chapter: [0.03] Study of Acids, Bases and Salts
Hydrogen chloride gas is prepared in the laboratory by the action of concentrated sulphuric acid on sodium chloride.
Give a balanced chemical equation for the above reaction.
Chapter: [0.084] Sulphuric Acid
Hydrogen chloride gas is prepared in the laboratory by the action of concentrated sulphuric acid on sodium chloride.
State the method of collection of the gas formed above.
Chapter: [0.084] Sulphuric Acid
Hydrogen chloride gas is prepared in the laboratory by the action of concentrated sulphuric acid on sodium chloride.
What is the property of sulphuric acid that makes it a suitable reagent for the reaction?
Chapter: [0.084] Sulphuric Acid
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CISCE previous year question papers ICSE Class 10 Chemistry with solutions 2023 - 2024
Previous year Question paper for CISCE ICSE Class 10 -2024 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CISCE ICSE Class 10 .
How CISCE ICSE Class 10 Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
