Advertisements
Advertisements
Question
The following sketch represents the electroplating of an Iron cup with Nickel metal.
Study the diagram and answer the following questions:
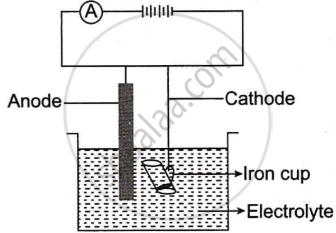
- During electroplating, the iron cup is placed at the cathode. Why?
- Name the ion that must be present in the electrolyte.
- State one condition that is necessary to ensure that the deposit is smooth, firm and even.
- Write the reaction taking place at the cathode.
- What change would you observe at the anode?
Solution
- In electroplating, the iron cup is put at the cathode because the cathode is a negative terminal that attracts metals that are positively charged. This leads to the reduction and formation of metal ions.
- The electrolyte used is a water-based solution of \[\ce{NiSO4}\], so the ions formed are \[\ce{Ni^{2+}, H+, SO_4^{2−}, OH−}\].
- To ensure smooth deposition, current should be passed slowly and over a longer period of time.
- Cathode: \[\ce{Ni^{2+} + 2e^- -> Ni}\] (Reduction)
- The anode, a Ni plate, is etched after the Ni ions finish in the electrolyte.
APPEARS IN
RELATED QUESTIONS
Give appropriate scientific reasons for Zinc oxide can be reduced to zinc metal by using
carbon, but aluminium oxide cannot be reduced by a reducing agent
Explain with reason:
In the electrolytic reduction of alumina, the graphite anode is gradually consumed.
Name oxide of one metal which is reduced by (give equation): Reduction with carbon
Give the chemical name and formula of 'cryolite'
what is the function of cryolite in the extraction of aluminium, other than acting as a solvent for bauxite?
4 tones of bauxite, 150 Kg of sodium hydroxide and 600 Kg of graphite.
The aluminium compound in bauxite is aluminium oxide and the main impurity is iron (II) oxide. Aluminium is obtained by the electrolysis of aluminium oxide dissolved in cryolite.
(a) When bauxite is treated with sodium hydroxide solution, what happens to:
(i) the aluminium oxide?
(ii) The iron (III) oxide?
Complete the following by selecting the correct option from the choices given :
The metal whose oxide, which is amphoteric, is reduced to metal by carbon reduction ________
Write the observation for the following:
A paper dipped in potassium permanganate solution is put on the mouth of a test tube containing sulphur dioxide gas.
Steel is an alloy of iron and ______.
Give the equations for the reduction of Lead (II) oxide.
