Advertisements
Advertisements
Question
Indicate the complex ion which shows geometrical isomerism.
Options
\[\ce{[Cr(H2O)4Cl2]^+}\]
\[\ce{[Pt(NH3)3Cl]}\]
\[\ce{[Co(NH3)6]^{3+}}\]
\[\ce{[Co(CN)5(NC)]^{3-}}\]
Solution
\[\ce{[Cr(H2O)4Cl2]^+}\]
Explanation:
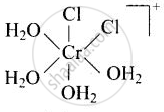
cis-isomer
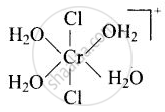
trans-isomer
APPEARS IN
RELATED QUESTIONS
Answer the following in one or two sentences.
Consider the complexes \[\ce{[Cu(NH3)4][PtCl4] and [Pt(NH3)4] [CuCl4]}\]. What type of isomerism these two complexes exhibit?
Define the term Co-ordination isomer.
Define the term Hydrated isomers.
The compound(s) that exhibit(s) geometrical isomerism is (are):
(I) [Pt(en)Cl2]
(II) [Pt(en)2]Cl2
(III) [Pt(en)2Cl2]
(IV) [Pt(NH3)2Cl2]
The number of geometrical isomers of [CrCl2(en)2]+ is ____________.
Which of the following does NOT show optical isomerism?
Which of the following shows maximum number of isomers?
The number of geometrical isomers of \[\ce{[Co(NH3)3 (NO3)3]}\] are ______.
Give cis isomer of [Co(NH3)4Cl2]⊕.
Write structures for geometrical isomers of diamminebromochloroplatinum (II).
