Advertisements
Advertisements
Question
Define the term Hydrated isomers.
Solution
Isomers in which there is exchange of solvent (water) ligands between coordination and ionization spheres are called hydrate isomers.
RELATED QUESTIONS
List various types of isomerism possible for coordination compounds, giving an example of each.
Why dextro and laevo rotatory isomers of Butan-2-ol are difficult to separate by fractional distillation?
The pair [Co(NH3)5(SO4)]Br and [Co(NH3)5Br]SO4 exhibits ____________ isomerism
Write the type of isomerism exhibited by [Co(NH3)5(NO2)]2+ and [Co(NH3)5ONO]2+ pair of complex ion.
Define the term Co-ordination isomer.
Draw structure of cis isomer of [Co(NH3)4Cl2]+
Draw optical isomers of [Co(en)3]3+.
Which one of the following pairs represents linkage isomers?
Which kind of isomerism is possible for a complex [Co(NH3)4Br2]Cl?
Draw all possible geometrical isomers of the complex \[\ce{[Co(en)2Cl2]^+}\] and identify the optically active isomer.
Explain optical isomerism in coordination compounds with an example.
Identify the CORRECT statement about the following complex of platinum.
[PtCl2(en)2]2+
Consider the two complexes given below:
\[\ce{\underset{(I)}{[Co(NH3)5SO4]Br}}\] and \[\ce{\underset{(II)}{[Co(NH3)5Br]SO4}}\]
I and II are ____________ isomers.
The formula of two complexes X and Y of chromium are given below:
\[\ce{\underset{(X)}{[Cr(H2O)6]Cl3}}\] and \[\ce{\underset{(Y)}{[Cr(H2O)5Cl]Cl2.H2O}}\]
X and Y are examples of ____________ isomers.
Which of the following has zero dipole moment?
How many donor groups are present in diethylene triamine?
Indicate the complex ion which shows geometrical isomerism.
Assertion: Complexes of MX6 and MX5L type (X and L are unidentate) do not show geometrical isomerism.
Reason: Geometrical isomerism is not shown by complexes of coordination number 6.
The relationship between compound (i) and (ii) is
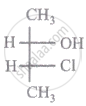 |
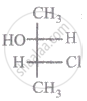 |
| (i) | (ii) |
Which of the following shows maximum number of isomers?
\[\ce{CH3CH2COO- Na+ ->[NaOH, + ?][Heat] CH3CH3 + Na2CO3}\]
Consider the above reaction and identify the missing reagent/chemical.
Write the name of isomerism in the following complexes:
[Cu(NH3)4] [PtCl4] and [Pt(NH3)4] [ CuCl4]
The number of geometrical isomers of \[\ce{[Co(NH3)3 (NO3)3]}\] are ______.
Give trans isomer of [Co(NH3)4Cl2]⊕.
Draw the geometrical isomers of the following complexes [Co(NH3)4Cl2]+
Draw the structure of trans isomers of Pt(NH3)2Cl2.
The co-ordination number of Co3+ ion in the complex [Co(NH3)4Cl2]⊕ is ______.
