Advertisements
Advertisements
Question
Draw optical isomers of [Co(en)3]3+.
Solution
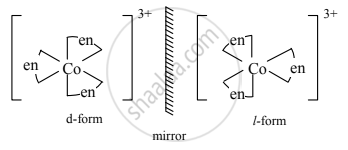
RELATED QUESTIONS
List various types of isomerism possible for coordination compounds, giving an example of each.
Choose the most correct option.
Which of the following complexes exist as cis and trans isomers?
1. [Cr(NH3)2Cl4]-
2. [Co(NH3)5Br]2⊕
3. [PtCl2Br2]2- (square planar)
4. [FeCl2(NCS)2]2- (tetrahedral)
Draw isomers of the following.
\[\ce{Pt(NH3)2ClNO2}\]
Draw the geometrical isomers of the following complexes [Pt(NH3)(H2O)Cl2].
Which one of the following will give a pair of enantiomorphs?
Which type of isomerism is exhibited by [Pt(NH3)2Cl2]?
How many geometrical isomers are possible for \[\ce{[Pt(Py)(NH3)(Br)(Cl)]}\]?
Fac-mer isomerism is shown by
What is linkage isomerism? Explain with an example.
Identify the CORRECT statement about the following complex of platinum.
[PtCl2(en)2]2+
The number of geometrical isomers of [CrCl2(en)2]+ is ____________.
Consider the two complexes given below:
\[\ce{\underset{(I)}{[Co(NH3)5SO4]Br}}\] and \[\ce{\underset{(II)}{[Co(NH3)5Br]SO4}}\]
I and II are ____________ isomers.
Which of the following is NOT an isomer of n-hexane?
What type of isomerism is present between (I) [Cr(H2O)6]Cl3 and (II) [Cr(H2O)5Cl]Cl2.H2O?
\[\ce{IUPAC}\] name of \[\ce{[Pt(NH3)2 Cl(NO2)]}\] is ______.
Assertion: Complexes of MX6 and MX5L type (X and L are unidentate) do not show geometrical isomerism.
Reason: Geometrical isomerism is not shown by complexes of coordination number 6.
Which of the following shows maximum number of isomers?
Write the name of isomerism in the following complexes:
[Cu(NH3)4] [PtCl4] and [Pt(NH3)4] [ CuCl4]
Draw geometric isomers of the following complex.
Geometrical isomers of Pt(NH3)2Cl2
Which of the following compound is optically active?
The one that is not expected to show isomerism is ______.
Which among the following solid is a non-polar solid?
Define Distereoisomers.
Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers: [Pt(NH3)(H2O)Cl2]
Draw the geometrical isomers of the following complexes [Co(NH3)4Cl2]+
Name the type of isomerism exhibited by the following pair of compound:
\[\ce{[Pt(NH3)4Cl2]Br2 and [Pt(NH3)4 Br2]Cl2}\]
Which one of the following complex ions has geometrical isomers?
