Advertisements
Advertisements
Question
Define Distereoisomers.
Solution
Two or more coordination compounds which contain the same number and types of atoms, and bonds (i.e., the connectivity between atoms is the same), but which have different spatial arrangements of the atoms are called distereoisomers.
APPEARS IN
RELATED QUESTIONS
Write the structural formula and IUPAC names of all possible isomers of the compound with molecular formula C3H8O.
Out of 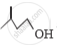 and
and 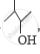 , which one is optically active and why ?
, which one is optically active and why ?
Why dextro and laevo rotatory isomers of Butan-2-ol are difficult to separate by fractional distillation?
Answer the following in one or two sentences.
Consider the complexes \[\ce{[Cu(NH3)4][PtCl4] and [Pt(NH3)4] [CuCl4]}\]. What type of isomerism these two complexes exhibit?
Answer in brief.
What are ionization isomers ? Give an example.
Define the term Hydrated isomers.
Draw optical isomers of [Co(en)3]3+.
How many geometrical isomers are possible for \[\ce{[Pt(Py)(NH3)(Br)(Cl)]}\]?
What is linkage isomerism? Explain with an example.
Which of the following is NOT a pair of structural isomers?
Consider the two complexes given below:
\[\ce{\underset{(I)}{[Co(NH3)5SO4]Br}}\] and \[\ce{\underset{(II)}{[Co(NH3)5Br]SO4}}\]
I and II are ____________ isomers.
____________ isomers are formed when the ligand has two different donor atoms.
The complex ions [Co(H2O)5(ONO)]2+ and [Co(H2O)5NO2]2+ are ____________.
The formula of two complexes X and Y of chromium are given below:
\[\ce{\underset{(X)}{[Cr(H2O)6]Cl3}}\] and \[\ce{\underset{(Y)}{[Cr(H2O)5Cl]Cl2.H2O}}\]
X and Y are examples of ____________ isomers.
Which of the following has zero dipole moment?
What is the number of moles of silver chloride precipitated when excess of aqueous silver nitrate is treated with [Co(NH3)4Cl2]Cl?
Assertion: Complexes of MX6 and MX5L type (X and L are unidentate) do not show geometrical isomerism.
Reason: Geometrical isomerism is not shown by complexes of coordination number 6.
Geometrical isomerism is not shown by
Complex [COCl2(en)2]+ can
Draw geometric isomers of the following complex.
Geometrical isomers of Pt(NH3)2Cl2
Which of the following are isostructural pairs?
(A) \[\ce{SO^{2-}4}\] and \[\ce{CrO^{2-}4}\]
(B) SiCl4 and TiCl4
(C) NH3 and \[\ce{NO^-3}\]
(D) BCl3 and BrCl3
Which compound would exhibit optical isomers?
Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers: [Pt(NH3)(H2O)Cl2]
Explain the ionisation isomers.
Draw the geometrical isomers of the following complexes [Co(NH3)4Cl2]+
Draw the structure of cis isomers of Pt(NH3)2Cl2.
Name the type of isomerism exhibited by the following pair of compound:
\[\ce{[Cr(H2O)5Cl]Cl2H2O and [Cr(H2O)4Cl2]Cl {.} 2H2O}\]
Which one of the following complex ions has geometrical isomers?
