Advertisements
Advertisements
Question
Name the type of isomerism exhibited by the following pair of compound:
\[\ce{[Cr(H2O)5Cl]Cl2H2O and [Cr(H2O)4Cl2]Cl {.} 2H2O}\]
Solution
Solvate isomerism
APPEARS IN
RELATED QUESTIONS
List various types of isomerism possible for coordination compounds, giving an example of each.
Choose the most correct option.
Which of the following complexes exist as cis and trans isomers?
1. [Cr(NH3)2Cl4]-
2. [Co(NH3)5Br]2⊕
3. [PtCl2Br2]2- (square planar)
4. [FeCl2(NCS)2]2- (tetrahedral)
Draw structure of cis isomer of [Co(NH3)4Cl2]+
Draw the geometrical isomers of the following complexes [Pt(NH3)(H2O)Cl2].
How many isomers are possible for an alkane having molecular formula C5H12?
The relationship between compound (i) and (ii) is
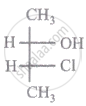 |
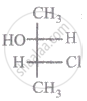 |
| (i) | (ii) |
Which of the following has an optical isomer?
Which of the following compound is optically active?
Explain the geometrical isomerism of the octahedral complex of the type [M(AA)2B2]n± with a suitable example.
Draw the geometrical isomers of the following complexes [Co(NH3)4Cl2]+
