Advertisements
Advertisements
Question
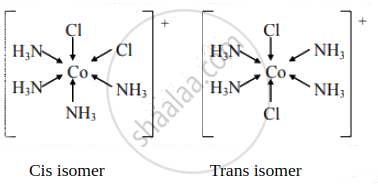
Draw the geometrical isomers of the following complexes [Co(NH3)4Cl2]+
Solution
RELATED QUESTIONS
Out of 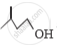 and
and 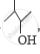 , which one is optically active and why ?
, which one is optically active and why ?
Give one chemical test as an evidence to show that [Co (NH3)5Cl] are ionisation isomers.
Answer the following in one or two sentences.
Predict whether the [Cr(en)2(H2O)2]3+ complex is chiral. Write the structure of its enantiomer.
Answer the following in one or two sentences.
Consider the complexes \[\ce{[Cu(NH3)4][PtCl4] and [Pt(NH3)4] [CuCl4]}\]. What type of isomerism these two complexes exhibit?
Draw isomers of the following.
\[\ce{Pt(NH3)2ClNO2}\]
Answer the following question.
Draw isomers of the following
[Cr(en2)Br2]⊕
Write the type of isomerism exhibited by [Co(NH3)5(NO2)]2+ and [Co(NH3)5ONO]2+ pair of complex ion.
Draw structure of cis isomer of [Co(NH3)4Cl2]+
Draw the geometrical isomers of the following complexes [Pt(NH3)(H2O)Cl2].
Draw optical isomers of [Co(en)3]3+.
Explain optical isomerism in coordination compounds with an example.
Which of the following is NOT a pair of structural isomers?
Identify the CORRECT statement about the following complex of platinum.
[PtCl2(en)2]2+
Which of the following does NOT show optical isomerism?
____________ isomers are formed when the ligand has two different donor atoms.
The complex ions [Co(H2O)5(ONO)]2+ and [Co(H2O)5NO2]2+ are ____________.
The formula of two complexes X and Y of chromium are given below:
\[\ce{\underset{(X)}{[Cr(H2O)6]Cl3}}\] and \[\ce{\underset{(Y)}{[Cr(H2O)5Cl]Cl2.H2O}}\]
X and Y are examples of ____________ isomers.
\[\ce{IUPAC}\] name of \[\ce{[Pt(NH3)2 Cl(NO2)]}\] is ______.
Which of the following compound show optical isomerism?
Write the name of isomerism in the following complexes:
[Cu(NH3)4] [PtCl4] and [Pt(NH3)4] [ CuCl4]
The number of geometrical isomers of \[\ce{[Co(NH3)3 (NO3)3]}\] are ______.
The one that is not expected to show isomerism is ______.
The compounds [PtCl2(NH3)4]Br2 and [PtBr2(NH3)4]Cl2 constitutes a pair of ______.
Give trans isomer of [Co(NH3)4Cl2]⊕.
Explain the geometrical isomerism of the octahedral complex of the type [M(AA)2B2]n± with a suitable example.
Draw the structure of trans isomers of Pt(NH3)2Cl2.
Name the type of isomerism exhibited by the following pair of compound:
\[\ce{[Cr(H2O)5Cl]Cl2H2O and [Cr(H2O)4Cl2]Cl {.} 2H2O}\]
Name the type of isomerism exhibited by the following pair of compound:
\[\ce{[Pt(NH3)4Cl2]Br2 and [Pt(NH3)4 Br2]Cl2}\]
