Advertisements
Advertisements
Question
Explain optical isomerism in coordination compounds with an example.
Solution
1. Coordination compounds which possess chirality exhibit optical isomerism similar to organic compounds.
2. The pair of two optically active isomers which are mirror images of each other are called enantiomers.
3. Their solutions rotate the plane of the plane polarised light either clockwise or anticlockwise and the corresponding isomers are called d (dextrorotatory) and 1 (levorotatory) forms respectively.
4. The octahedral complexes of type [M(xx)3]n±, [M(xx)2AB]n± and [M(xx)2B2]n± exhibit optical isomerism.
Examples:
1. The optical isomers of [Co(en)3]3+ are shown below.

Optical isomer
2. The coordination complex [COCl2(en)2]+ has three isomers, two optically active cis forms and one optically inactive transform. These structures are shown below.

Optical isomers
3. In a coordination compound of type [PtCl2(en)2]2+, two geometrical isomers are possible. They are cis and trans. Among these two isomers, cis isomer shows optically active isomerism because the whole molecule is asymmetric.
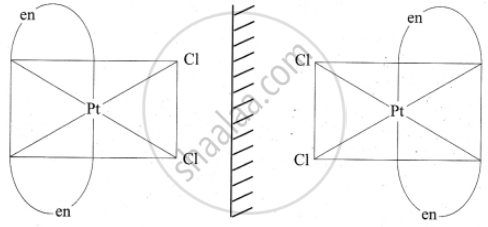
Optical isomers of Cis [PtCl2(en)2]2+
APPEARS IN
RELATED QUESTIONS
Write the structural formula and IUPAC names of all possible isomers of the compound with molecular formula C3H8O.
Choose the most correct option.
Which of the following complexes exist as cis and trans isomers?
1. [Cr(NH3)2Cl4]-
2. [Co(NH3)5Br]2⊕
3. [PtCl2Br2]2- (square planar)
4. [FeCl2(NCS)2]2- (tetrahedral)
Answer the following in one or two sentences.
Predict whether the [Cr(en)2(H2O)2]3+ complex is chiral. Write the structure of its enantiomer.
Answer the following question.
Draw geometric isomers and enantiomers of the following complex.
[Pt(en)2ClBr]2⊕
The pair [Co(NH3)5(SO4)]Br and [Co(NH3)5Br]SO4 exhibits ____________ isomerism
Draw optical isomers of [Co(en)3]3+.
Which of the following is NOT a pair of structural isomers?
Assertion: Complexes of MX6 and MX5L type (X and L are unidentate) do not show geometrical isomerism.
Reason: Geometrical isomerism is not shown by complexes of coordination number 6.
Write structures for geometrical isomers of diamminebromochloroplatinum (II).
Draw the structure of trans isomers of Pt(NH3)2Cl2.
