Advertisements
Advertisements
Question
Draw isomers of the following.
\[\ce{Pt(NH3)2ClNO2}\]
Solution
Cis and trans isomers of \[\ce{Pt(NH3)2ClNO2}\]
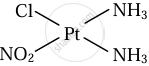 |
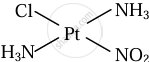 |
| Cis isomer | Trans isomer |
APPEARS IN
RELATED QUESTIONS
Write the structural formula and IUPAC names of all possible isomers of the compound with molecular formula C3H8O.
List various types of isomerism possible for coordination compounds, giving an example of each.
Out of 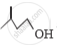 and
and 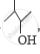 , which one is optically active and why ?
, which one is optically active and why ?
Give one chemical test as an evidence to show that [Co (NH3)5Cl] are ionisation isomers.
Choose the most correct option.
Which of the following complexes exist as cis and trans isomers?
1. [Cr(NH3)2Cl4]-
2. [Co(NH3)5Br]2⊕
3. [PtCl2Br2]2- (square planar)
4. [FeCl2(NCS)2]2- (tetrahedral)
Answer the following in one or two sentences.
Consider the complexes \[\ce{[Cu(NH3)4][PtCl4] and [Pt(NH3)4] [CuCl4]}\]. What type of isomerism these two complexes exhibit?
Answer the following question.
Draw isomers of the following
Ru(NH3)4Cl2
Draw all possible geometrical isomers of the complex \[\ce{[Co(en)2Cl2]^+}\] and identify the optically active isomer.
Explain optical isomerism in coordination compounds with an example.
What are hydrate isomers? Explain with an example.
Identify the CORRECT statement about the following complex of platinum.
[PtCl2(en)2]2+
The formula of two complexes X and Y of chromium are given below:
\[\ce{\underset{(X)}{[Cr(H2O)6]Cl3}}\] and \[\ce{\underset{(Y)}{[Cr(H2O)5Cl]Cl2.H2O}}\]
X and Y are examples of ____________ isomers.
\[\ce{IUPAC}\] name of \[\ce{[Pt(NH3)2 Cl(NO2)]}\] is ______.
Assertion: Complexes of MX6 and MX5L type (X and L are unidentate) do not show geometrical isomerism.
Reason: Geometrical isomerism is not shown by complexes of coordination number 6.
Which of the following does not show optical isomerism?
Which of the following has an optical isomer?
\[\ce{CH3CH2COO- Na+ ->[NaOH, + ?][Heat] CH3CH3 + Na2CO3}\]
Consider the above reaction and identify the missing reagent/chemical.
Draw geometric isomers of the following complex.
Geometrical isomers of Pt(NH3)2Cl2
The number of geometrical isomers of \[\ce{[Co(NH3)3 (NO3)3]}\] are ______.
White precipitate of AgCl dissolves in aqueous ammonia solution due to formation of ______.
The compounds [PtCl2(NH3)4]Br2 and [PtBr2(NH3)4]Cl2 constitutes a pair of ______.
Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers: [Pt(NH3)(H2O)Cl2]
Explain the geometrical isomerism of the octahedral complex of the type [M(AA)2B2]n± with a suitable example.
Explain the ionisation isomers.
