Advertisements
Advertisements
प्रश्न
Draw isomers of the following.
\[\ce{Pt(NH3)2ClNO2}\]
उत्तर
Cis and trans isomers of \[\ce{Pt(NH3)2ClNO2}\]
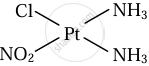 |
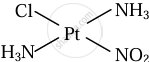 |
| Cis isomer | Trans isomer |
APPEARS IN
संबंधित प्रश्न
List various types of isomerism possible for coordination compounds, giving an example of each.
Out of 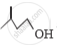 and
and 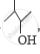 , which one is optically active and why ?
, which one is optically active and why ?
Give one chemical test as an evidence to show that [Co (NH3)5Cl] are ionisation isomers.
Choose the most correct option.
Which of the following complexes exist as cis and trans isomers?
1. [Cr(NH3)2Cl4]-
2. [Co(NH3)5Br]2⊕
3. [PtCl2Br2]2- (square planar)
4. [FeCl2(NCS)2]2- (tetrahedral)
The pair [Co(NH3)5(SO4)]Br and [Co(NH3)5Br]SO4 exhibits ____________ isomerism
Define the term Hydrated isomers.
Which type of isomerism is exhibited by [Pt(NH3)2Cl2]?
How many geometrical isomers are possible for \[\ce{[Pt(Py)(NH3)(Br)(Cl)]}\]?
Which one of the following pairs represents linkage isomers?
Which one of the following complexes is not expected to exhibit isomerism?
What is linkage isomerism? Explain with an example.
How many isomers are possible for an alkane having molecular formula C5H12?
The number of geometrical isomers of [CrCl2(en)2]+ is ____________.
____________ isomers are formed when the ligand has two different donor atoms.
\[\ce{IUPAC}\] name of \[\ce{[Pt(NH3)2 Cl(NO2)]}\] is ______.
The relationship between compound (i) and (ii) is
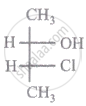 |
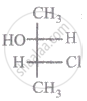 |
| (i) | (ii) |
Which of the following has an optical isomer?
White precipitate of AgCl dissolves in aqueous ammonia solution due to formation of ______.
The compounds [PtCl2(NH3)4]Br2 and [PtBr2(NH3)4]Cl2 constitutes a pair of ______.
Give cis isomer of [Co(NH3)4Cl2]⊕.
Indicate the type of isomerism exhibited by the following complex and draw the structures for this isomer:
\[\ce{[Pt(NH3)(H2O)Cl2]}\]
Explain the ionisation isomers.
Draw the structure of cis isomers of Pt(NH3)2Cl2.
Draw the structure of trans isomers of Pt(NH3)2Cl2.
The co-ordination number of Co3+ ion in the complex [Co(NH3)4Cl2]⊕ is ______.
Name the type of isomerism exhibited by the following pair of compound:
\[\ce{[Pt(NH3)4Cl2]Br2 and [Pt(NH3)4 Br2]Cl2}\]
