Advertisements
Advertisements
प्रश्न
Name the type of isomerism exhibited by the following pair of compound:
\[\ce{[Pt(NH3)4Cl2]Br2 and [Pt(NH3)4 Br2]Cl2}\]
उत्तर
Ionisation isomerism
APPEARS IN
संबंधित प्रश्न
Why dextro and laevo rotatory isomers of Butan-2-ol are difficult to separate by fractional distillation?
Draw isomers of the following.
\[\ce{Pt(NH3)2ClNO2}\]
Which type of isomerism is exhibited by [Pt(NH3)2Cl2]?
What are hydrate isomers? Explain with an example.
Identify the CORRECT statement about the following complex of platinum.
[PtCl2(en)2]2+
How many isomers are possible for an alkane having molecular formula C5H12?
Assertion: Complexes of MX6 and MX5L type (X and L are unidentate) do not show geometrical isomerism.
Reason: Geometrical isomerism is not shown by complexes of coordination number 6.
The relationship between compound (i) and (ii) is
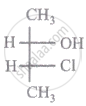 |
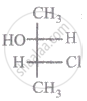 |
| (i) | (ii) |
Which of the following has an optical isomer?
The number of geometrical isomers of \[\ce{[Co(NH3)3 (NO3)3]}\] are ______.
