Advertisements
Advertisements
Question
Answer the following question.
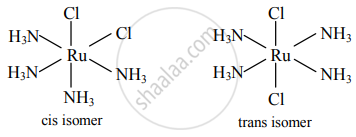
Draw isomers of the following
Ru(NH3)4Cl2
Solution
Cis and trans isomers of Ru(NH3)4Cl2
APPEARS IN
RELATED QUESTIONS
Write the structural formula and IUPAC names of all possible isomers of the compound with molecular formula C3H8O.
Give one chemical test as an evidence to show that [Co (NH3)5Cl] are ionisation isomers.
Choose the most correct option.
Which of the following complexes exist as cis and trans isomers?
1. [Cr(NH3)2Cl4]-
2. [Co(NH3)5Br]2⊕
3. [PtCl2Br2]2- (square planar)
4. [FeCl2(NCS)2]2- (tetrahedral)
Draw isomers of the following.
\[\ce{Pt(NH3)2ClNO2}\]
Answer the following question.
Draw geometric isomers and enantiomers of the following complex.
[Pt(en)2ClBr]2⊕
The pair [Co(NH3)5(SO4)]Br and [Co(NH3)5Br]SO4 exhibits ____________ isomerism
Write the type of isomerism exhibited by [Co(NH3)5(NO2)]2+ and [Co(NH3)5ONO]2+ pair of complex ion.
How many geometrical isomers are possible for \[\ce{[Pt(Py)(NH3)(Br)(Cl)]}\]?
Which one of the following complexes is not expected to exhibit isomerism?
Draw all possible geometrical isomers of the complex \[\ce{[Co(en)2Cl2]^+}\] and identify the optically active isomer.
Explain optical isomerism in coordination compounds with an example.
What are hydrate isomers? Explain with an example.
Which would exhibit coordination isomerism?
Which of the following does NOT show optical isomerism?
How many donor groups are present in diethylene triamine?
What type of isomerism is present between (I) [Cr(H2O)6]Cl3 and (II) [Cr(H2O)5Cl]Cl2.H2O?
Indicate the complex ion which shows geometrical isomerism.
The relationship between compound (i) and (ii) is
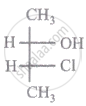 |
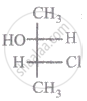 |
| (i) | (ii) |
Draw geometric isomers of the following complex.
Geometrical isomers of Pt(NH3)2Cl2
Which compound would exhibit optical isomers?
What are structural isomers or constitutional isomers?
Match the pairs in column I (pairs of isomers) and column II (types of isomers)
| Column I (Pairs of isomers) |
Column II (Types of isomers) |
| (A) [Cr(H2O)5Cl]Cl2.H2O and [Cr(H2O)4Cl2]Cl.2H2O | (i) Ionization isomers |
| (B) [Co(en)2(NO2)2]+ and [Co(en)2(ONO2)]+ | (ii) Hydrate isomers |
| (C) [Co(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [Co(CN)6] | (iii) Linkage isomers |
| (D) [Pt(NH3)4Cl2] Br2 and [Pt(NH3)4Br2]Cl2 | (iv) Coordination isomers |
Draw the geometrical isomers of the following complexes [Co(NH3)4Cl2]+
Draw the structure of trans isomers of Pt(NH3)2Cl2.
The co-ordination number of Co3+ ion in the complex [Co(NH3)4Cl2]⊕ is ______.
Name the type of isomerism exhibited by the following pair of compound:
\[\ce{[Cr(H2O)5Cl]Cl2H2O and [Cr(H2O)4Cl2]Cl {.} 2H2O}\]
